Novel synthesis method of calcipotriol
A technology of calcipotriol and its synthetic method, which is applied in the new technical field of calcipotriol synthesis, can solve the problems of unindustrialized reports, etc., and achieve the effects of short route, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
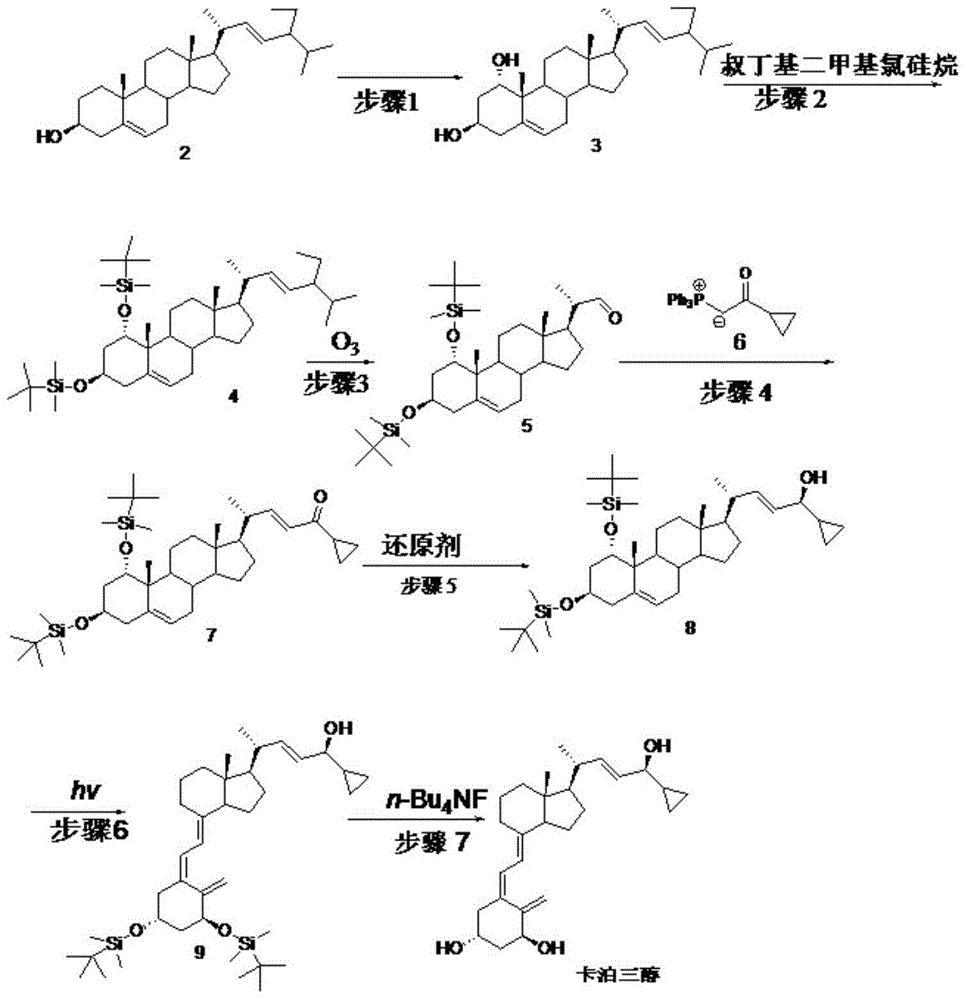
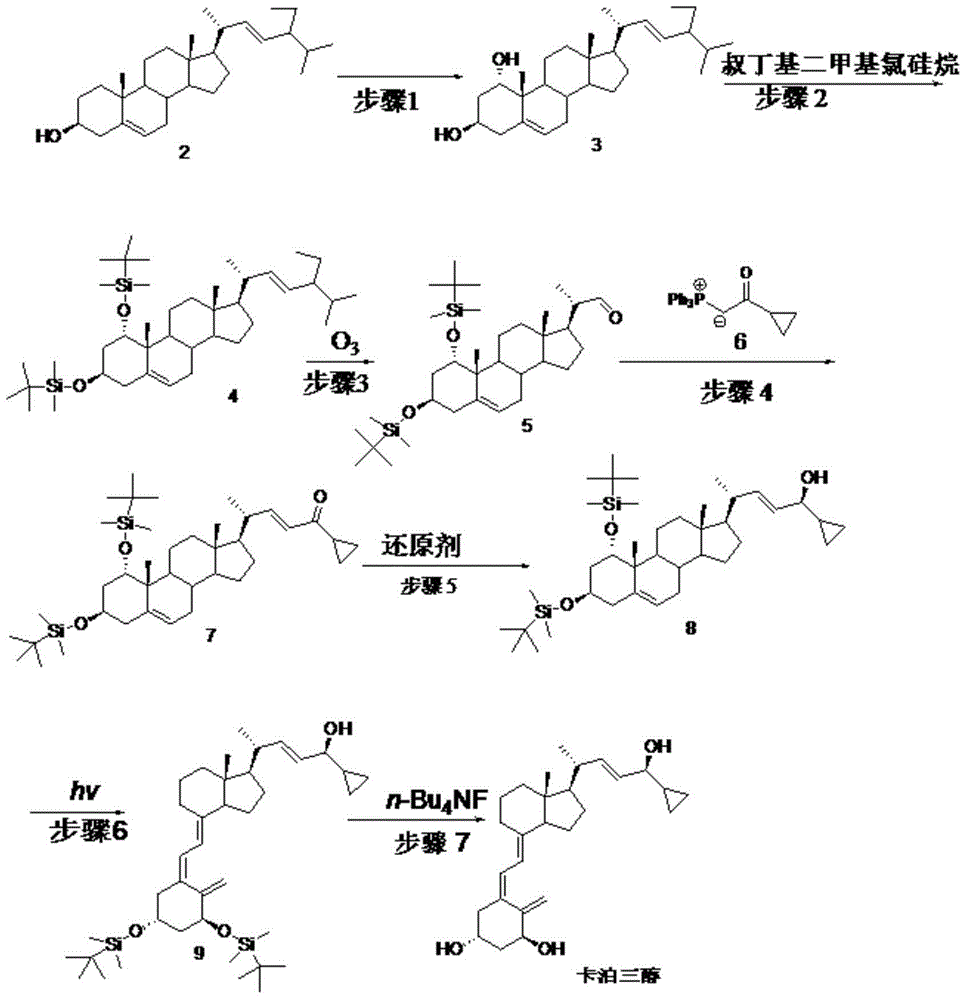
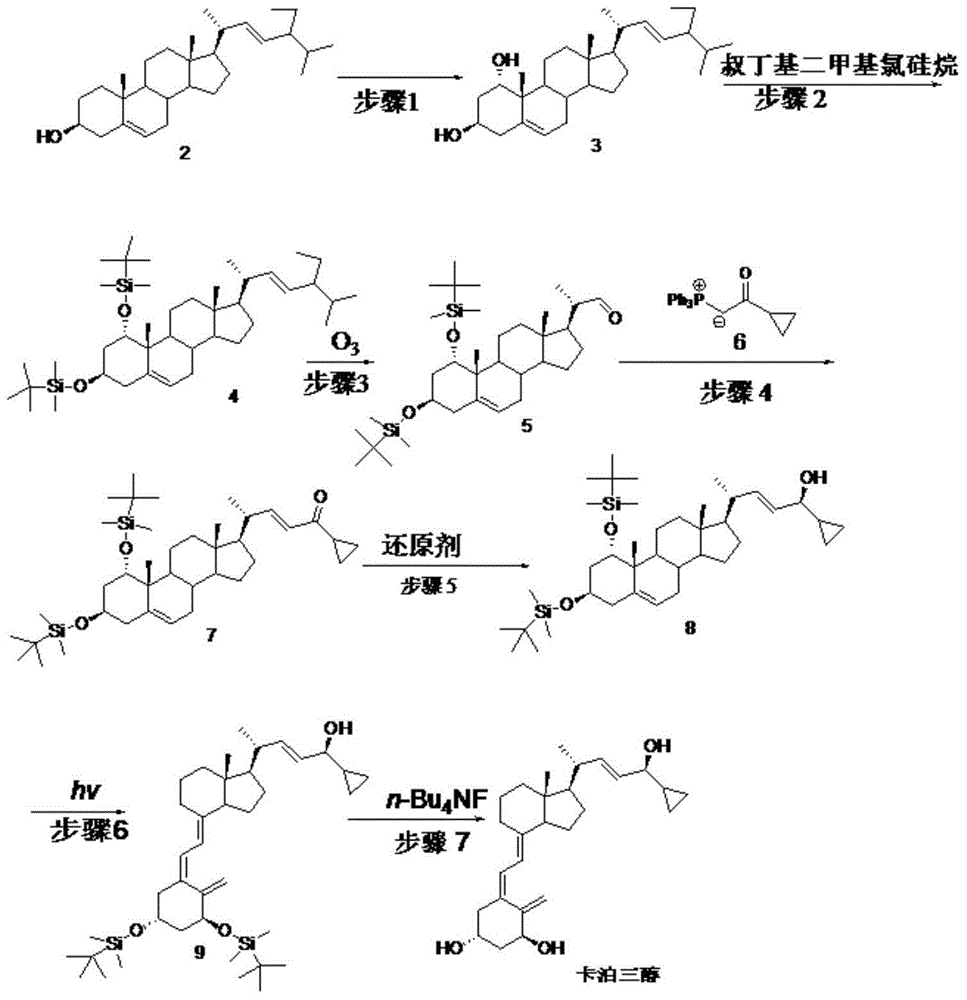
[0069] Embodiment 1: step 1 biological hydroxylation
[0070] Embodiment 1-1 put 100ml containing glucose 1%, corn steep liquor 1%, potassium dihydrogen phosphate 0.05%, the culture medium of pH6.5 (hydrochloric acid adjustment) is put into fermenter, inserts Penicillium oxalicum P.oxalicumAS3 after sterilization .7766 seed solution, ventilated and stirred, cultivated at 26.5°C for 2 days, added compound 2 to 0.1% Tween 80 solution to make a suspension, and added it to the fermenter, the substrate feeding concentration was 5g / L, continued After 3 days of fermentation, after the reaction was terminated, the fermentation broth was extracted with ethyl acetate, and the layers were left to stand. The organic layer was concentrated under reduced pressure to obtain the crude product of compound 3, and then repeatedly recrystallized with a mixed solvent of toluene and chloroform to obtain the refined product of compound 3 (content 95 %, yield 55%).
[0071] Embodiment 1-2 100ml cont...
Embodiment 2
[0074] Example 2: Step 2
[0075] Example 2-1 Compound 3 (42.9g, 0.1mol) was dissolved in 200ml of DMF, then imidazole (29.9g, 0.44mol) and TBDMSCl (33.2g, 0.22mol) were added, stirred at 15°C for 2 hours, and concentrated under reduced pressure A minimum amount was poured into 50ml of ethanol, cooled to room temperature and diluted in ice water, filtered, the filter cake was washed with water and then dried to obtain 61.6g of compound 4 with a yield of 95.8%.
[0076] Example 2-2 Compound 3 (42.9g, 0.1mol) was dissolved in 200ml of DMSO, then imidazole (29.9g, 0.44mol) and TBDMSCl (33.2g, 0.22mol) were added, stirred at 35°C for 1.5 hours, then concentrated under reduced pressure A minimum amount was poured into 50ml of ethanol, cooled to room temperature and diluted in ice water, filtered, the filter cake was washed with water and then dried to obtain 62.5g of compound 4 with a yield of 95.2%.
[0077] Example 2-3 Compound 3 (42.9g, 0.1mol) was dissolved in 200mlTHF, then t...
Embodiment 3
[0079] Example 3: Step 3
[0080] Example 3-1 Compound 4 (65.7g, 0.1mol) was added to pyridine (200ml), stirred and dissolved, then cooled to -35°C, and O 3 gas, undergoes an oxidation reaction. TLC followed the reaction and stopped feeding O 3 gas, into which N 2 Will O 3 After cleaning, it was raised to room temperature, washed successively with saturated sodium bicarbonate solution and water, and concentrated to dryness under reduced pressure to obtain light yellow oily compound 5 (48.2 g). (yield 83.9%)
[0081] Example 3-2 Compound 4 (65.7g, 0.1mol) and triethylamine (50ml) were added to chloroform (500ml), stirred and dissolved, then cooled to -15°C, and O 3 gas, undergoes an oxidation reaction. TLC followed the reaction and stopped feeding O 3 gas, into which N 2 Will O 3 After cleaning, it was raised to room temperature, washed successively with saturated sodium bicarbonate solution and water, and then concentrated to dryness under reduced pressure to obtain l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com