Preparation method of psoriasis therapeutic medicine calcipotriol
A technology of calcipotriol and therapeutic drugs, which is applied in the field of preparation of calcipotriol, a drug for treating psoriasis, can solve the problems of product yield, poor quality, high degradation rate, etc., achieve less impurities, low degradation rate, good yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
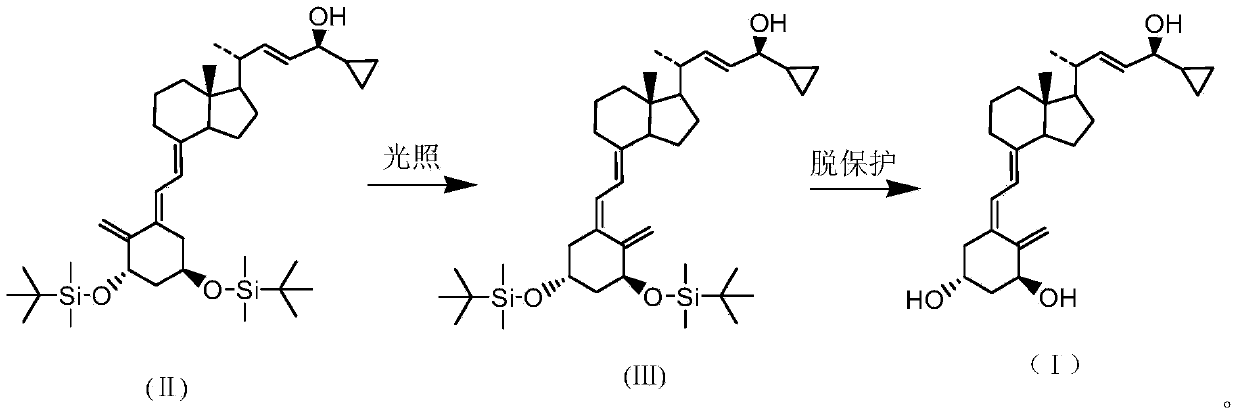
[0016] Preparation: (5Z,7E,22E,24S)-24-cyclopropyl-1α,3β-bis[[(1,1-dimethyl)tert-butylsilyl]oxy]-9,10-ring-opened cholesteric -5,7,10(19),22-tetraen-24-ol
[0017] Put 50g (5E,7E,22E,24S)-24-cyclopropyl-1,3-bis[[(1,1-dimethyl)tert-butylsilyl]oxygen]-9,10 into the photochemical reaction apparatus - Ring-opened cholesteryl-5,7,10(19),22-tetraen-24-ol, 1.3ml of toluene, 3g of anthracene, 0.5ml of triethylamine, protected by nitrogen gas. Lower the temperature to -5°C, and control the light wavelength to 250-265nm to carry out the light reaction for 45 minutes. After the reaction was complete, it was filtered and concentrated to dryness in vacuo to obtain 50.2 g of the title compound in the form of white foam.
Embodiment 2
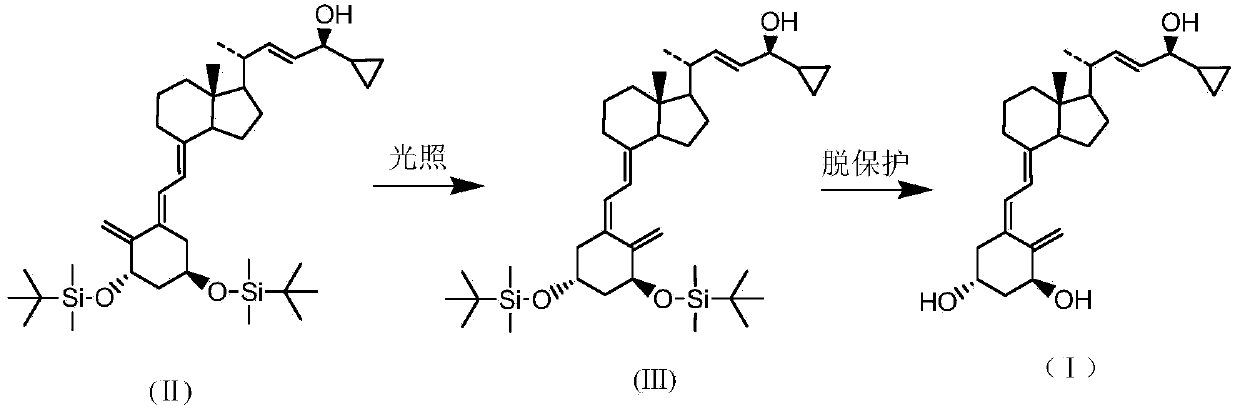
[0019] Preparation: (5Z,7E,22E,24S)-24-cyclopropyl-1α,3β-bis[[(1,1-dimethyl)tert-butylsilyl]oxy]-9,10-ring-opened cholesteric -5,7,10(19),22-tetraen-24-ol
[0020] Put 50g (5E,7E,22E,24S)-24-cyclopropyl-1,3-bis[[(1,1-dimethyl)tert-butylsilyl]oxygen]-9,10 into the photochemical reaction apparatus - Ring-opened cholestan-5,7,10(19),22-tetraen-24-ol, xylene 1.3ml, anthracene 3g, triethylamine 0.5ml, nitrogen protection. Lower the temperature to -5°C, and control the light wavelength to 250-265nm to carry out the light reaction for 45 minutes. After the reaction was complete, it was filtered and concentrated to dryness in vacuo to obtain 50.7 g of the title compound in the form of white foam.
Embodiment 3
[0022] Preparation: (5Z,7E,22E,24S)-24-cyclopropyl-9,10-cyclocholesta-5,7,10(19),22-tetraene-1α,3β,24-triol
[0023] The foam obtained in Example 1 above was completely dissolved with tetrahydrofuran, 104 g of phosphomolybdic acid / silicon dioxide was added, and the temperature was raised to 60° C. for 3 hours to react. After filtration, the filtrate was concentrated to dryness in vacuo, and 5 L of methyl formate was added to reflux to dissolve completely, then cooled to -10°C, kept for crystallization for 2 hours, and filtered to obtain 21.5 g of a white crystalline powder of the title compound, with a yield of 66.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com