Method for separating and determining calcipotriol midbody L and related impurities
A technology for calcipotriol and intermediates, which is applied in the field of analytical chemistry, can solve the problems of not separating and measuring intermediates, etc., and achieves the effects of effective impurity control, high accuracy, and separation and measurement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 related impurity M b , M b The 20S isomer, M c , M c Localization of the 20S isomer
[0049] (1) M b , M b The 20S isomer, M c , M c Preparation of the 20S isomer localization solution
[0050] m b Positioning solution: weigh the impurity M b 15.11mg, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, that is.
[0051] m c Positioning solution: weigh the impurity M c 15.26mg, put it in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, that is.
[0052] m b 20S isomer localization solution: Weigh out the impurity M b 15.28 mg of the 20S isomer, put in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and you get it.
[0053] m c 20S isomer localization solution: Weigh out the impurity M c Put 15.22mg of 20S isomer in a 50ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and you get it.
[0054] (2) ...
Embodiment 2
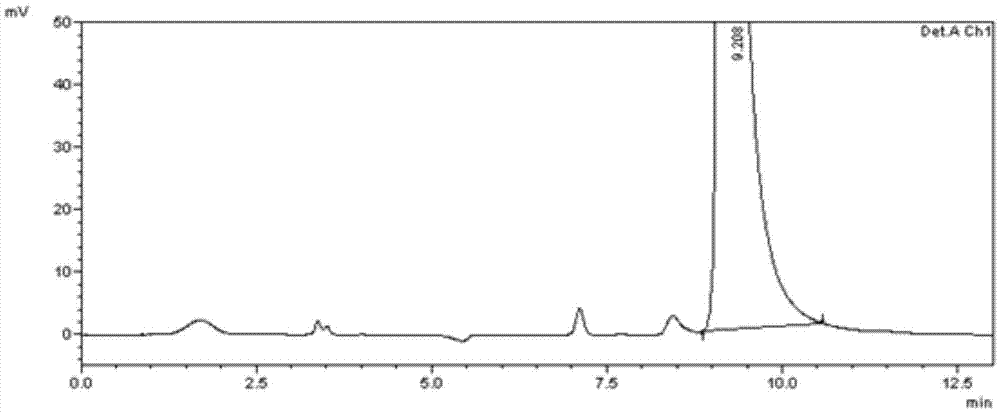
[0056] Example 2 Calcipotriol intermediate L and related impurities M b , M b The 20S isomer, M c , M c Separation of the 20S isomer
[0057] (1) Intermediate L and related impurities M b , M b The 20S isomer, M c , M c Preparation of mixed solution of 20S isomers
[0058] Accurately pipette the M prepared in Example 1 respectively b , M c , M b The 20S isomer and the M c Put 3.3ml of each 20S isomer positioning solution in the same 100ml measuring bottle, add diluent to dilute to the mark, shake well to get a mixed positioning stock solution, then accurately weigh 10.26mg of intermediate L reference substance and put it in a 10ml measuring bottle , add 1.0ml of mixed positioning stock solution, then add diluent to dissolve and dilute to the mark, shake well to obtain a mixed solution.
[0059] (2) Take 20 μ l of the mixed solution for sample injection, carry out high-performance liquid chromatography analysis according to the above-mentioned chromatographic condit...
Embodiment 3
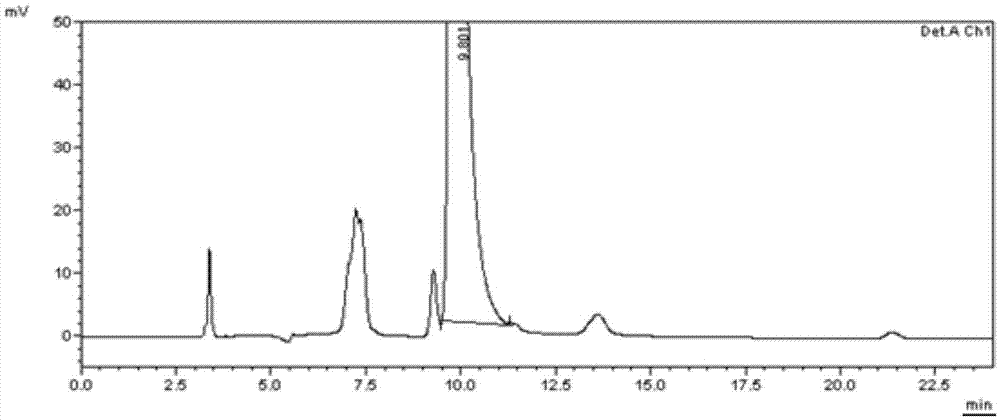
[0060] The separation and detection of embodiment 3 need testing product
[0061] (1) Preparation of the test solution: Take about 10 mg of the test product, put it in a 10 ml measuring bottle, add a diluent to dissolve it and dilute it to the mark, shake well, and you get it.
[0062] (2) Get need testing solution, mixed solution 20 μ l sample injections respectively, carry out high performance liquid chromatography analysis according to above-mentioned chromatographic condition, record chromatogram, the chromatogram of need testing solution is as follows Figure 7 As shown, no related impurity M was detected in the test solution. b , M b The 20S isomer, M c and M c The 20S isomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com