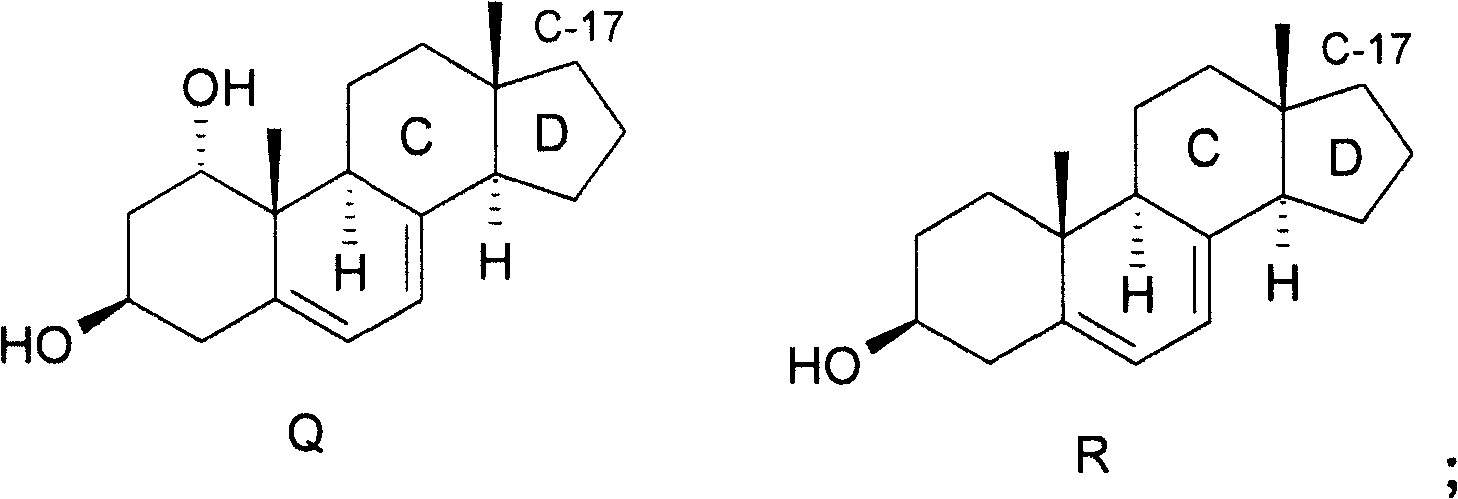
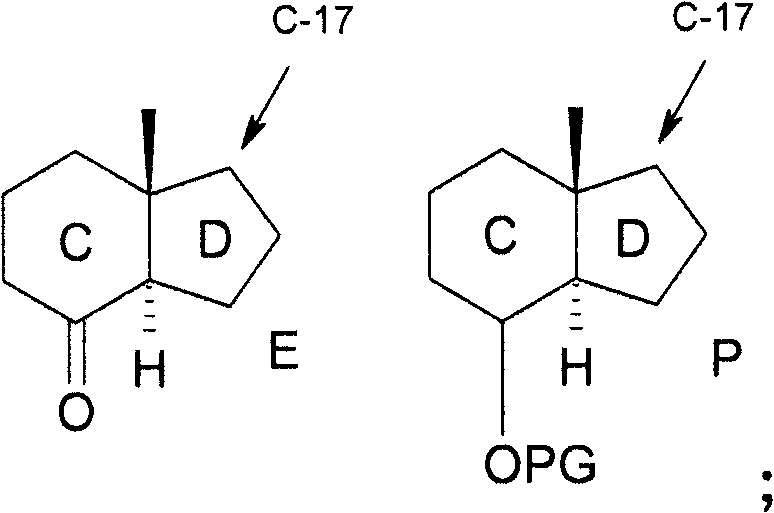
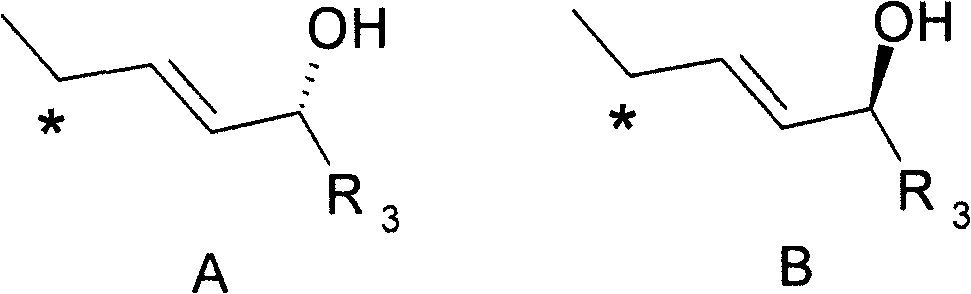Epimerisation of allylic alcohols
一种差向异构化、差向异构体的技术,应用在合成维生素D类似物的化合物领域,能够解决经济不利、没有公开醇差向异构化等问题,达到易于操作、改善总产率和方法生产率、避免酯化和皂化步骤的效果
Inactive Publication Date: 2010-09-08
LEO PHARMA AS
View PDF4 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
This epimerization process has the disadvantage that it involves two additional chemical transformations, esterification and saponification of IIIb, making the process economically unfavorable, especially on an industrial scale
WO94 / 07853 discloses various vitamin D analogs having a hydroxyl substituent on the asymmetric allyl carbon at position 24, but does not disclose a method for epimerizing the alcohol
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment approach
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The present invention relates to processes for epimerising alcohols of compounds having a hydroxyl substituent on an asymmetric allylic carbon, such as compounds useful for the synthesis of vitamin D analogues where the epimeric hydroxyl substituent is at the 24 position. The invention further relates to methods of producing intermediates useful for the synthesis of calcipotriol by said epimerisation processes.
Description
field of invention The present invention relates to a novel method of epimerization of compounds, such as those useful in the synthesis of vitamin D analogues, which have a hydroxyl substituent on an asymmetric allylic carbon, for example at position 24. The present invention further relates to the application of the intermediate produced by this method in the preparation of calcipotriol i.e. {(5Z, 7E, 22E, 24S)-24-cyclopropyl-9,10-broken cholesterol-5,7,10(19 ), 22-tetraene-1α-3β-24-triol} or calcipotriol monohydrate. Background of the invention Calcipotriol (Structure I) [CAS 112965-21-6] shows strong activity in inhibiting undesirable proliferation of epidermal keratinocytes [F.A.C.M. Castelijins, M.J. Gerritsen, I.M.J.J.van Vlijmen-Willems, P.J. van Erp, P.C.M. van de Kerkhof; Acta Derm. Venereol. 79, 11, 1999]. The efficacy of calcipotriol and monohydrate (I-hydrate) calcipotriol in the treatment of psoriasis has been shown in many clinical trials [D.M.Ashcroft et al...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C401/00C07J9/00
CPCC07J9/00C07C2101/02C07C401/00C07C2601/02
Inventor H·佩得森C·A·S·布雷丁E·T·宾德鲁普
Owner LEO PHARMA AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com