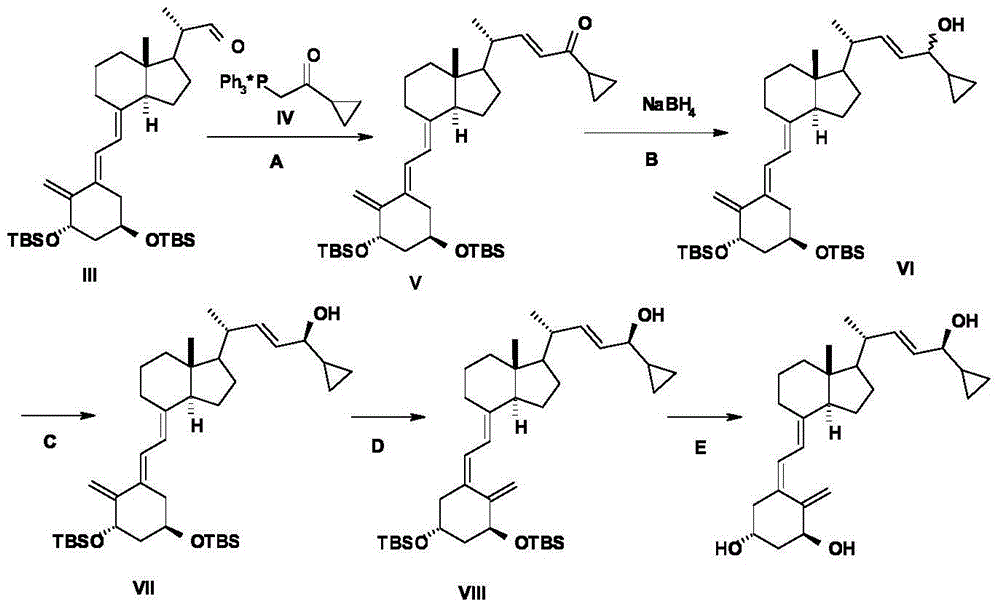
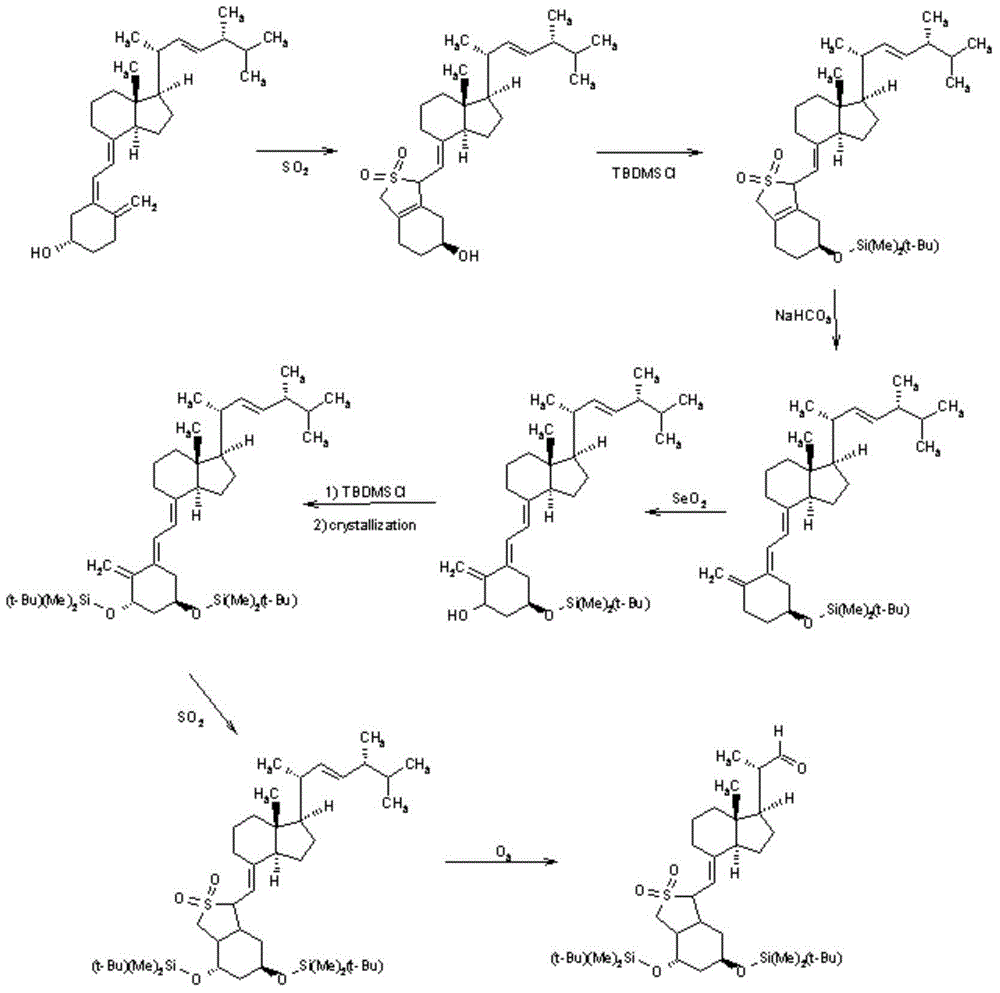
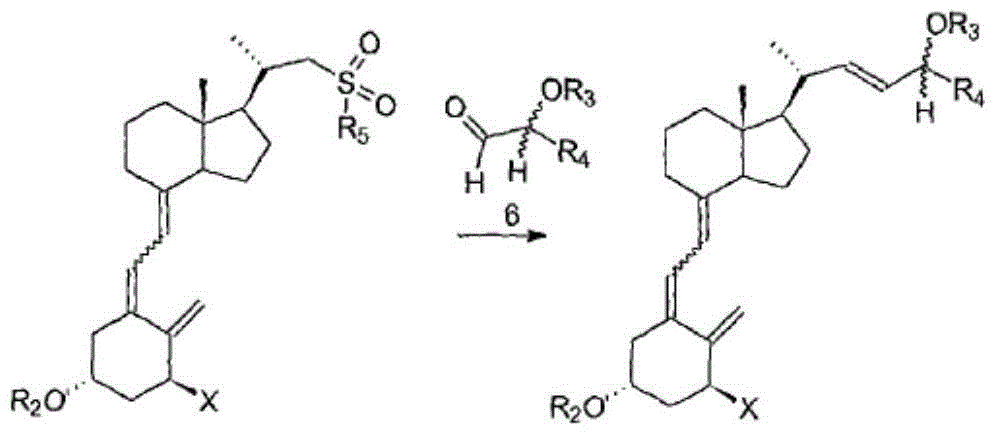
Preparation method of calcipotriol
A technology based on calcipotriol and triisopropylsilyl, applied in the field of preparation of calcipotriol
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Preparation of (1S, 2R)-1-cyclopropyl-2-(p-tolylsulfoxide)ethanol
[0100] Reaction formula:
[0101]
[0102] Steps:
[0103] 100 ml three-necked flask, add 25 ml tetrahydrofuran, add 0.655 g zinc chloride and 0.89 g (2R)-(p-tolylsulfoxide)-1-cyclopropyl ketone, stir and mix well at 20°C, cool to - Below 80°C, through the dropping funnel, add 12 ml of DIBAL-H toluene solution (1M) dropwise within 30 minutes, keep the temperature not higher than -80°C, stir for 1 hour, then quench the reaction system with 5 ml of methanol Remove the solvent by rotary evaporation, add 8 milliliters of 10 milliliters of water and 2.5 mol / liter of sodium hydroxide, and extract the system three times with dichloromethane, each 30 milliliters, the oil layers are combined, dried over anhydrous sodium sulfate, and the solvent is removed by rotary evaporation, 0.7 g of the product was obtained, which could be directly used in the next hydroxyl protection step; yield: 77%, de=98....
Embodiment 2
[0106] Example 2: Preparation of (1S, 2R)-1-cyclopropyl-2-(p-tolylsulfoxide)ethanol
[0107] 100 ml three-necked flask, add 20 ml 1,4-dioxane, add 0.81 g zinc bromide and 0.67 g (2R)-(p-tolylsulfoxide)-1-cyclopropyl ketone, stir and mix at 20°C Evenly, drop the temperature to below -90°C, drop 10 ml of DIBAL-H toluene solution (1M) within 30 minutes through the dropping funnel, keep the temperature not higher than -90°C, stir the reaction for 1 hour, and then the reaction system Quenched with 5 ml of methanol; the solvent was removed by rotary evaporation, 10 ml of water and 6 ml of 2.5 mol / L sodium hydroxide were added, the system was extracted three times with dichloromethane, 30 ml each time, the oil layers were combined, and dried over anhydrous sodium sulfate , and the solvent was removed by rotary evaporation to obtain 0.47 g of the product, which can be directly used in the next hydroxyl protection step; yield: 74%, de=98.6.
Embodiment 3
[0108] Example 3: Preparation of (1S, 2R)-1-cyclopropyl-2-(p-tolylsulfoxide)ethanol
[0109] 100 ml three-necked flask, add 20 ml of anisole, add 1.01 g of zinc bromide and 0.67 g of (2R)-(p-tolylsulfoxide)-1-cyclopropyl ketone, stir and mix at 20°C, and cool down with To below -50°C, through the dropping funnel, add 5 ml of DIBAL-H toluene solution (1M) dropwise within 30 minutes, keep the temperature not higher than -50°C, stir for 3 hours, then the reaction system is washed with 5 ml of methanol Quenching; the solvent was removed by rotary evaporation, 6 ml of 10 milliliters of water and 2.5 mol / liter of sodium hydroxide were added, the system was extracted three times with toluene, 30 milliliters each time, the oil layers were combined, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation, Obtain 0.47 g of the product, which can be directly used in the next hydroxyl protection step; yield: 74%, de=98.3 (1S, 2R)-1-cyclopropyl-2-(p-tolylsulf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com