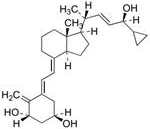
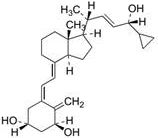
Method for detecting procalcipotriol, impurity C and impurity D in calcipotriol ointment
A technology of calcipotriol and detection method, which is applied in the field of analysis and detection, can solve problems such as the effective separation of unmentioned calcipotriol, and achieve the effect of ensuring safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Determination according to high performance liquid chromatography (Chinese Pharmacopoeia 2020 edition general rule 0512).
[0045] Chromatographic conditions:
[0046] High-performance liquid chromatography is adopted, and the filler of the chromatographic column is octadecylsilane bonded silica gel; the column length of the chromatographic column is 250mm, the diameter is 4.6mm, and the filler particle size is 5μm;
[0047] The flow rate through the chromatographic column is 1.0 mL / min, the injection volume is 20 μL, and the column temperature is 30°C;
[0048] The detector used in the analysis and determination is selected from the ultraviolet detector, and the detection wavelength of the ultraviolet detector is 264nm;
[0049] Using isocratic elution, the mobile phase includes mobile phase A and mobile phase B, mobile phase A is water, mobile phase B is acetonitrile, and the volume ratio of mobile phase A and mobile phase B is 45:55.
[0050] Assay:
[0051] Solut...
Embodiment 2
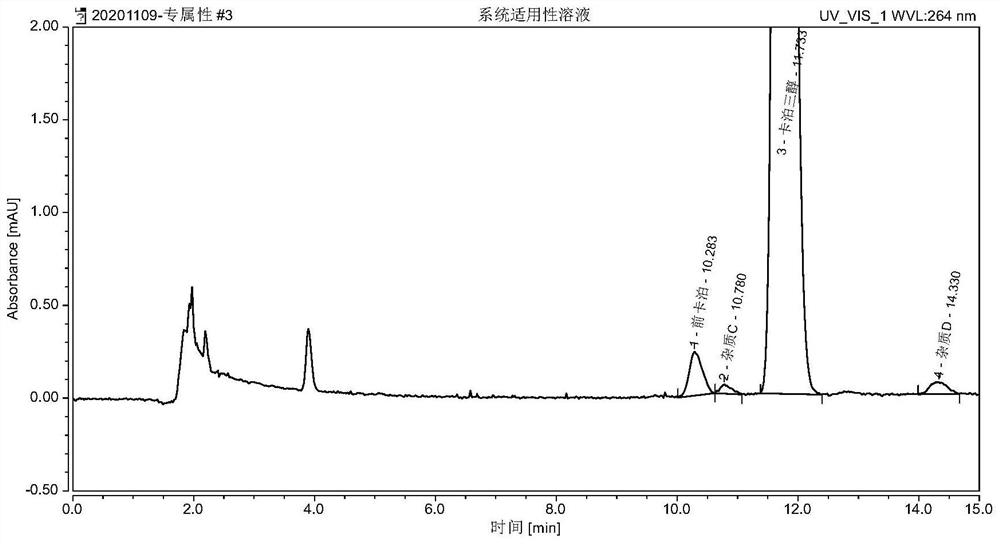
[0052] Example 2 Detecting the system suitability solution test of procalcipotriol, impurity C and impurity D in calcipotriol ointment
[0053] Take the appropriate amount of reference substances such as impurity C, impurity D and calcipotriol, dissolve and dilute with mobile phase to make a mixed solution containing 0.005 mg of impurity C, 0.005 mg of impurity D and 1 mg of calcipotriol per 1 mL, shake well, As a system suitability solution. Precisely measure 50 μL, inject it into the liquid chromatograph, and record the chromatogram. The peaks of pre-calcipotriol, impurity C, calcipotriol and impurity D appear in sequence, and the separation between adjacent chromatographic peaks is not less than 1.5. The retention time of each component is as figure 1 As shown, the test results are as follows:
[0054] components Retention time (min) relative retention time Separation procalcipotriol 10.283 0.88 - Impurity C 10.780 0.92 1.60 Calcipotri...
Embodiment 3
[0056] Example 3 Quantitation limit test of procalcipotriol, impurity C and impurity D in a kind of calcipotriol ointment
[0057] Take the appropriate amount of impurity C, impurity D and calcipotriol reference substance respectively, place them in a volumetric flask, dissolve them with a diluent and make a solution containing 10 ng of impurity C, impurity D and calcipotriol per mL, and use The diluent was gradually diluted until the S / N was no less than 10.0, and the solution with this concentration was used as the limit of quantitation solution, and six parallel preparations were made. Precisely measure 50 μL of each solution, inject them into the liquid chromatograph respectively, and record the chromatograms. The test results are as follows:
[0058] name Quantitation limit solution (μg / mL) Concentration level (%) Impurity C 0.0024 0.04 Impurity D 0.0025 0.04 Calcipotriol 0.0025 0.04
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Column length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com