Ir (III)-based chiral metal-organic porous material with splitting function as well as preparation method and application of Ir (III)-based chiral metal-organic porous material
A porous material, chiral technology, applied in organic chemistry methods, preparation of organic compounds, organic racemization, etc., can solve problems such as limiting the resolution effect, and achieve the effect of good application prospects, efficient resolution, and high yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
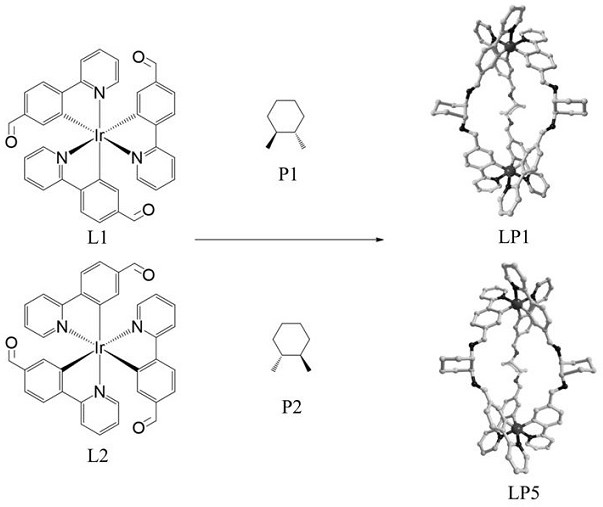
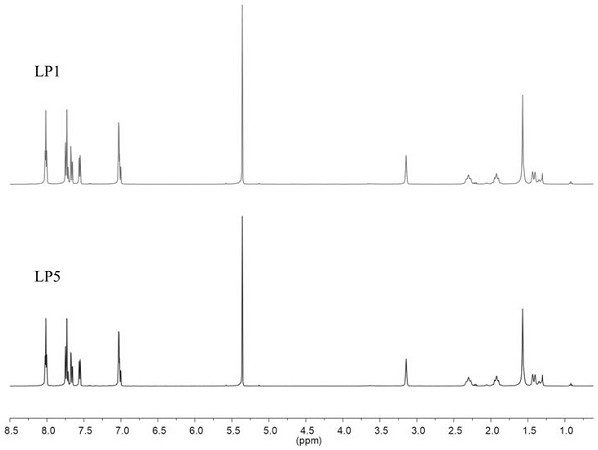
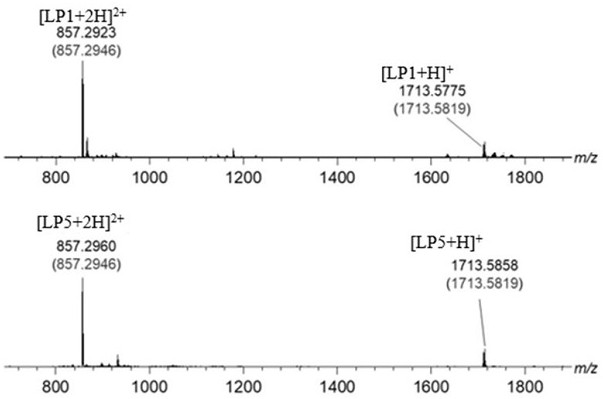
[0043] Synthesis of the target compound LP1: the Λ - fac -Ir-CHO (37.0 mg , 0.05 mmol) and 1 S ,2 S -Cyclohexanediamine (11.4 mg, 0.10 mmol) was placed in a 50 mL three-necked flask, to which was added 30 mL of toluene and acetonitrile solvent with a volume ratio of 1:2, stirred to dissolve completely, and then 15 mol% p-toluene was added Sulfonic acid was reacted under argon atmosphere at 110 °C for 12 h. After the reaction solution was cooled to room temperature, it was distilled under reduced pressure to obtain an orange-yellow solid, which was dissolved in dichloromethane and diffused with ether to obtain crystalline LP1, 38.5 mg, yield 90 %. Theoretical value of elemental analysis [C 90 h 78 Ir 2 N 12 ·H 2 O·(C 7h 8 )]: H, 4.87; C, 69.93; N, 9.22. The experimental values are H, 4.81; C, 61.68; N, 9.54. The obtained target material structure is as follows figure 1 As shown, the NMR spectrum is shown as figure 2 As shown, the mass spectrogram is as image...
Embodiment 2
[0045] Synthesis of the target compound LP3: the Λ - fac -Ir-CHO (37.0 mg, 0.05 mmol) and (S)-(-)-diaminopropane (7.4 mg, 0.10 mmol) were placed in a 50 mL three-necked flask, and 35 mL of Toluene and acetonitrile solvent, stir to dissolve completely, then add 15 mol% p-toluenesulfonic acid, react under argon atmosphere at 100 °C for 13.5 h, after the reaction solution is cooled to room temperature, distill under reduced pressure to obtain an orange-yellow solid, which is dissolved in In dichloromethane, diethyl ether was diffused to obtain crystalline LP3, 36.6 mg, with a yield of 92%. The obtained target material LP3 structure was identified by mass spectrometry, HRMS-ESI m / z calcd for C 81 h 67 Ir 2 N 12 [M+H] + 1593.4878 found 1598.4831
Embodiment 3
[0047] Synthesis of the target compound LP4: the Λ - fac -Ir-CHO (37.0 mg, 0.05 mmol) and (1S,2S)-1,2-diphenylethylenediamine (25.4 mg, 0.12mmol) were placed in a 50 mL three-necked flask, and 45 mL volume ratio of Toluene and acetonitrile solvent of 1:2.5, stirred to dissolve completely, then added 15 mol% p-toluenesulfonic acid, reacted under argon atmosphere at 120 ℃ for 10h, after the reaction solution was cooled to room temperature, it was distilled under reduced pressure to obtain an orange-yellow solid, It was dissolved in dichloromethane and diffused with ether to obtain crystalline LP4, 46.5 mg, with a yield of 93%. The structure of the obtained target material LP4 was identified by mass spectrometry, HRMS-ESI m / z calcd for C 114 h 85 Ir 2 N 12 [M+H] + 2007.6298 found 2007.6200.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com