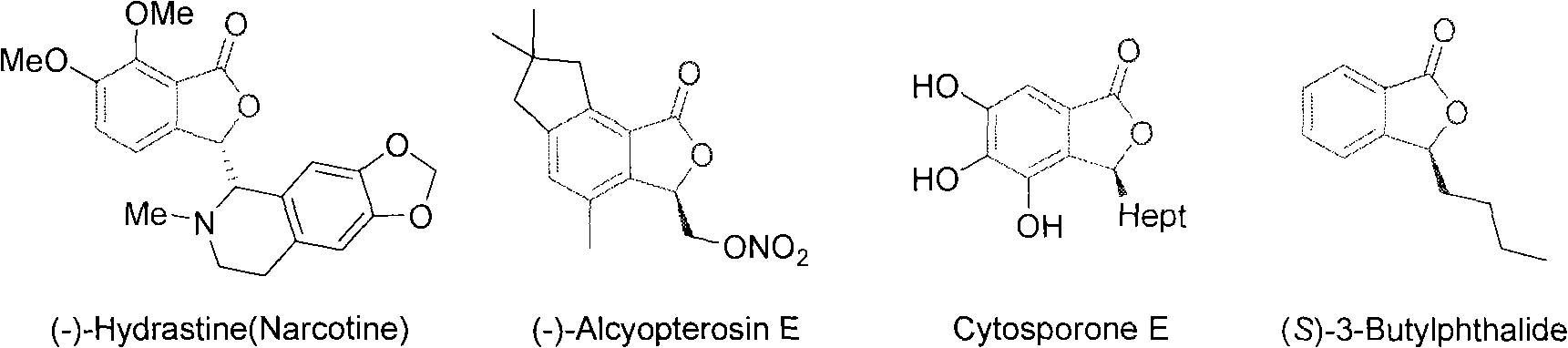
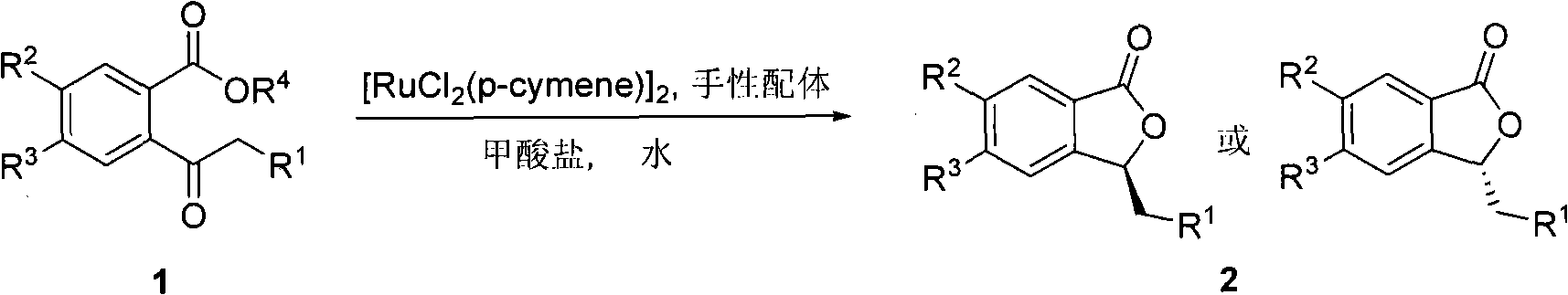
Method for preparing high optical purity 3-substituted chiral phthalide compounds
A technology of optical purity and phthalides, which is applied in the field of preparing 3-substituted chiral phthalides with high optical purity, can solve the problems of side reactions, not very mature, and low selectivity, and achieve high selectivity and bottom good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 2a
[0033] In a 5mL Schlenk bottle, add 0.0025mmol [RuCl 2 (p-cymene)] 2, 0.006mmol chiral diamine ligand, change argon protection, add 1.0mL distilled water, and stir at a certain temperature for 0.5 to 1 hour. Under the condition of increasing argon flow, open the lid and add 2.5mmol formate and 0.5mmol substrate, under the protection of argon, stir the reaction at a certain temperature, and process for 20 hours. Add 5ml of distilled water, extract with ethyl acetate, dry over anhydrous sodium sulfate, concentrate, and purify by flash silica gel column chromatography to obtain the corresponding phthalide compound. Refer to Table 1 for the yield and ee value (negative value indicates that the product configuration is reversed).
[0034]
[0035] Table 1
[0036]
Embodiment 2
[0038] Synthesis of 2a
[0039] In a 5mL Schlenk bottle, add 0.0025mmol [RuCl 2 (p-cymene)] 2 , 0.006 mmol of ligand (S, S)-11, under argon protection, 1.0 mL of distilled water was added, and the reaction was stirred at 40° C. for 0.5 to 1 hour. Under the condition of increasing argon flow, open the lid and add 5mmol sodium formate, 0.04mmol surfactant, 0.5mmol substrate, under the protection of argon, stir the reaction at 40°C, and react for 20 hours. Add 5ml of distilled water, extract with ethyl acetate, dry over anhydrous sodium sulfate, concentrate, and purify by flash silica gel column chromatography to obtain the corresponding phthalide compound 2a. The conversion rate of the reaction is shown in Table 2.
[0040]
[0041] Table 2
[0042]
Embodiment 3
[0044] Synthesis of 2a
[0045] In a 5mL Schlenk bottle, add 0.0025mmol [RuCl 2 (p-cymene)] 2 , 0.006 mmol of ligand (S, S)-11, under argon protection, 1.0 mL of distilled water was added, and the reaction was stirred at 40° C. for 0.5 to 1 hour. Under the condition of increasing argon flow, open the lid and add 5mmol sodium formate, 0.04mmol CTAB, 1mmol substrate, and stir the reaction at 40°C under the protection of argon until the nuclear magnetic resonance shows that the conversion is complete. Added 5ml of distilled water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated, and purified by flash silica gel column chromatography to obtain the corresponding phthalide compound 2a with a yield of 92% and an ee of 98.3%.
[0046]
[0047] 2a: 1 H NMR (300MHz, CDCl 3 ): δ0.91(t, J=7.2Hz, 3H), 1.33-1.51(m, 4H), 1.71-1.81(m, 1H), 2.00-2.09(m, 1H), 5.49(dd, J=4.2 , 7.8Hz, 1H), 7.45(dd, 0.75, 7.7Hz, 1H), 7.53(t, J=7.5Hz, 1H), 7.68(dt, J=0.9, 7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com