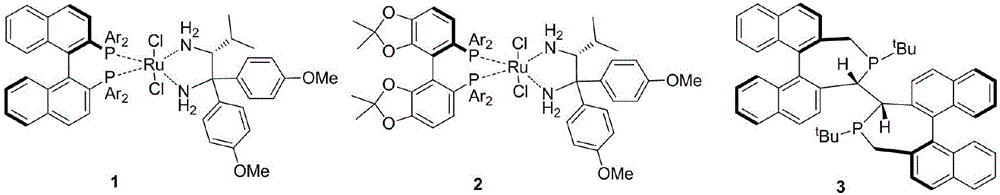
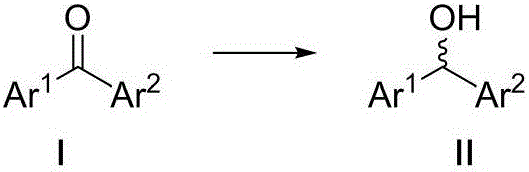
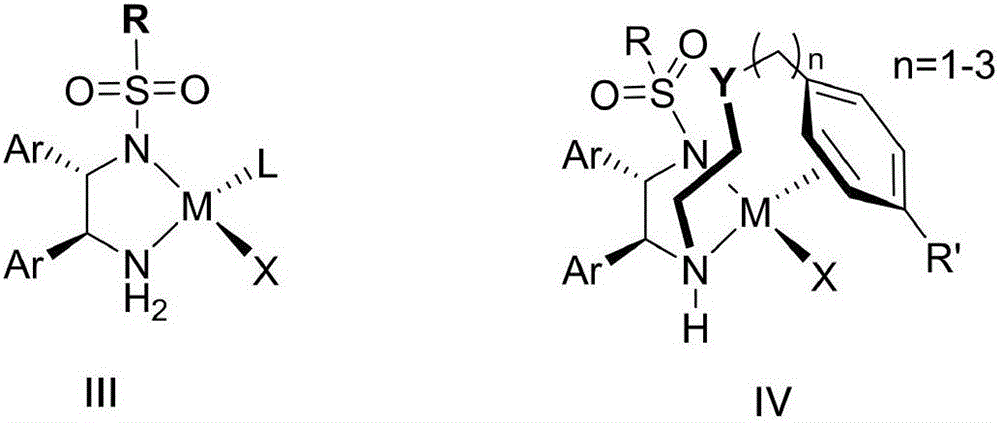
Optical activity di(heteto)aryl methanol and asymmetric synthesis method thereof
An optically active, aryl methanol technology, applied in asymmetric synthesis, organic chemistry methods, chemical instruments and methods, etc., to achieve the effects of low price, mild reaction conditions, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Catalyst A-I catalyzes the asymmetric synthesis of (2-methylphenyl) (2-pyridyl) methanol
[0030] Add 0.01 mmol of catalysts numbered A-I to 10 ml of Schlenk test tubes, add 0.2 mmol (2-methylphenyl) (2-pyridyl) ketone, 2 mmol sodium formate, seal the test tube, and replace the gas with nitrogen for 3 times , add 1 mL DMSO / HO by syringe 2 O (1:1) mixed solvent, reacted at 50°C for 24 hours. After the reaction was completed, it was extracted 3 times with ethyl acetate, combined and concentrated to dryness, and analyzed by nuclear magnetic resonance 1 The reaction conversion was determined by HNMR, and the enantiomeric excess ee value of the product (2-methylphenyl)(2-pyridyl)methanol was determined by HPLC. The results are shown in Table 1. HPLC separation conditions: chiral column Daicel AD-H column, mobile phase: n-hexane / isopropanol=95:5 (volume ratio), flow rate: 1.0 ml / min, wavelength: 254 nm, column temperature: 30 degrees Celsius, t 1 = 13.70 m...
Embodiment 2
[0033] Example 2: Asymmetric synthesis of (2-methylphenyl)(2-pyridyl)methanol in different solvents
[0034] Add 0.01 mmol of catalyst A to a 10 ml Schlenk test tube, add 0.2 mmol (2-methylphenyl) (2-pyridyl) ketone, 2 mmol sodium formate, seal the test tube, replace the gas with nitrogen for 3 times, and add it with a syringe 1 ml of solvent was reacted at 50°C for 24 hours. After the reaction was completed, it was extracted 3 times with ethyl acetate, combined and concentrated to dryness, and analyzed by nuclear magnetic resonance 1 The reaction conversion was determined by H NMR, and the enantiomeric excess ee value of the product (2-methylphenyl)(2-pyridyl)methanol was determined by HPLC. The results are shown in Table 2.
[0035] Table 2 Catalyst A-I catalyzes the asymmetric synthesis of (2-methylphenyl) (2-pyridyl) methanol
[0036]
Embodiment 3
[0037] Embodiment 3: Taking formic acid / triethylamine as the asymmetric synthesis of hydrogen source (2-methylphenyl)(2-pyridyl)methanol
[0038] 0.01mmol catalyst A is added respectively in the Schlenk test tube of 10 milliliters, adds 0.2mmol (2-methylphenyl) (2-pyridyl) ketone, 1 milliliter of formic acid / triethylamine (molar ratio 1.1 / 1), Seal the test tube, replace the gas with nitrogen three times, and react at 50°C for 24 hours. After the reaction was completed, water was added, extracted 3 times with ethyl acetate, combined and concentrated to dryness, and analyzed by nuclear magnetic resonance 1 The reaction conversion rate was 63% as measured by HNMR, and the enantiomeric excess ee value of the product (2-methylphenyl)(2-pyridyl)methanol as determined by HPLC was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com