Pentaerythritol loaded chiral diamine derivative thiourea catalyst, preparation method and application thereof
A thiourea catalyst and pentaerythritol technology are applied in the preparation of carbamate derivatives, catalytic reactions, organic chemical methods, etc., can solve the problems of difficult product separation, expensive thiourea catalyst, troublesome recovery, etc., and achieve high yield and synthesis. Simple route and constant catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
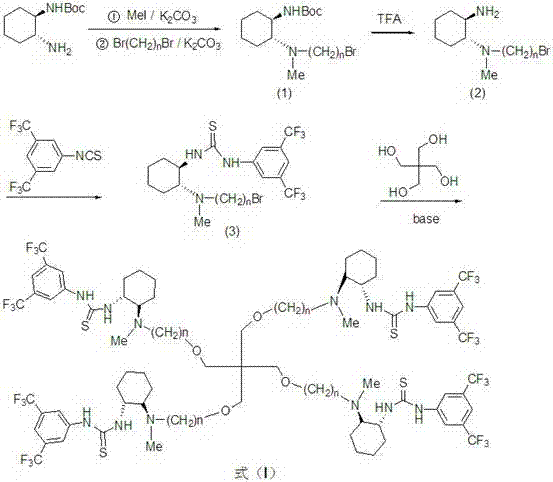
[0025] Preparation of intermediate (1):
[0026] In a 25mL flask, add 7mL CH 3 CN, 214mg (1mmol) ( S,S )- N -Boc-cyclohexanediamine, 331 mg (2.4 mmol) K 2 CO 3 and 156 mg (1.1 mmol) CH 3 I, react at room temperature for 36 hours, then add 238 mg (1.1 mmol) of 1,4-dibromobutane, continue to react at room temperature for 48 hours, filter, concentrate, and purify by column chromatography to obtain intermediate (1) with a yield of 93%.
Embodiment 2
[0028] Preparation of intermediate (2):
[0029] 363 mg (1 mmol) of intermediate (1) was added to 171 mg (1.5 mmol) of trifluoroacetic acid in CH 2 Cl 2 (4mL) mixed solution, stirred at room temperature for 1h, added 30uL of Et 3 N, stirred for 0.5h, evaporated to dryness under reduced pressure, and purified by column chromatography to obtain intermediate (2) with a yield of 95%.
Embodiment 3
[0031] Preparation of intermediate (3):
[0032] Dissolve 263 mg (1 mmol) of intermediate (2) in 5 mL CH 2 Cl 2 In, add 165mg (1.2mmol) K 2 CO 3 , stirred at room temperature, added dropwise 325 mg (1.2 mmol) of 3,5-bis(trifluoromethyl)isothiocyanate in CH 2 Cl 2 (3mL) solution, reacted at room temperature for 8h, filtered, concentrated, purified by column chromatography (V CH2Cl2 :V CH3OH =20:1), the intermediate (3) was obtained with a yield of 98%. IR (CHCl 3 ): 1723cm -1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com