Fluorescent probe for identifying cysteine and glutathione as well as preparation method and application thereof
A technology of glutathione and cysteine, applied in the field of chemical analysis and detection, can solve the problem of not having the function of targeting lysosomes, and achieve the effects of high sensitivity, mild reaction conditions and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of intermediates
[0052] React with 3-carboxy-4-chloro-7-diethylaminocoumarin and 3-hydroxy-N-(2-ethylmorpholine) benzamide as raw materials to obtain an intermediate, and the specific process includes:
[0053] Step 1: Add anhydrous dichloromethane (5mL) to 3-carboxy-4-chloro-7-diethylaminocoumarin (295mg, 1mmol) and stir to dissolve, then add oxalyl chloride (0.9mL, 10mmol) in sequence , anhydrous DMF (5 μ L), reacted at room temperature under the protection of argon for 2.5 hours, distilled off the solvent under reduced pressure, then added anhydrous dichloromethane to dissolve (5 mL), and obtained the first reaction solution;
[0054] Step 2. Add 3-hydroxy-N-(2-ethylmorpholine) benzamide (1mmol) and anhydrous triethylamine (4.4mL, 30mmol) into anhydrous dichloromethane (5mL) and stir to dissolve to obtain the Second reaction solution;
[0055] Step 3: Add the first reaction solution to the second reaction solution at ℃, react in an ice-water bath for ...
Embodiment 2
[0057] Preparation of fluorescent probes:
[0058] The specific process of obtaining the probe by reacting the intermediate and p-nitrophenol as raw materials is as follows:
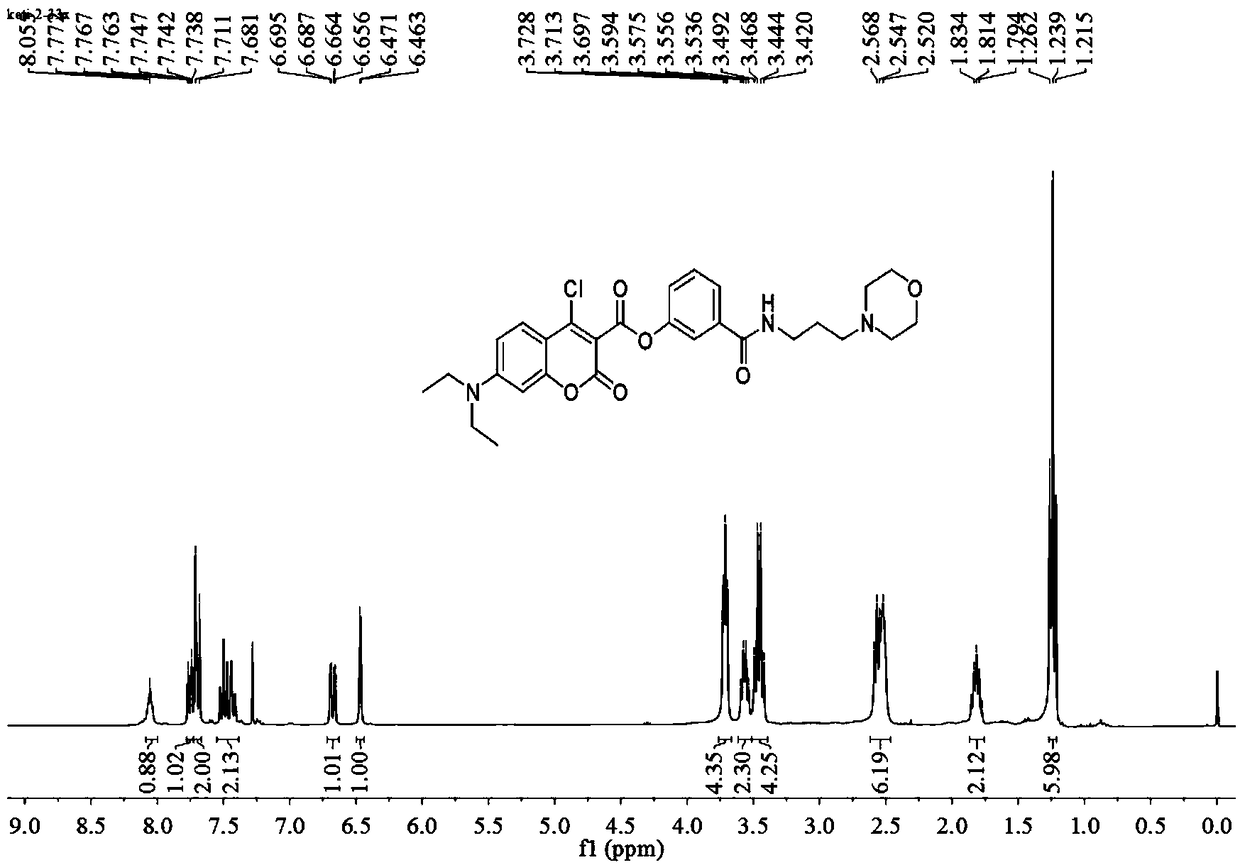
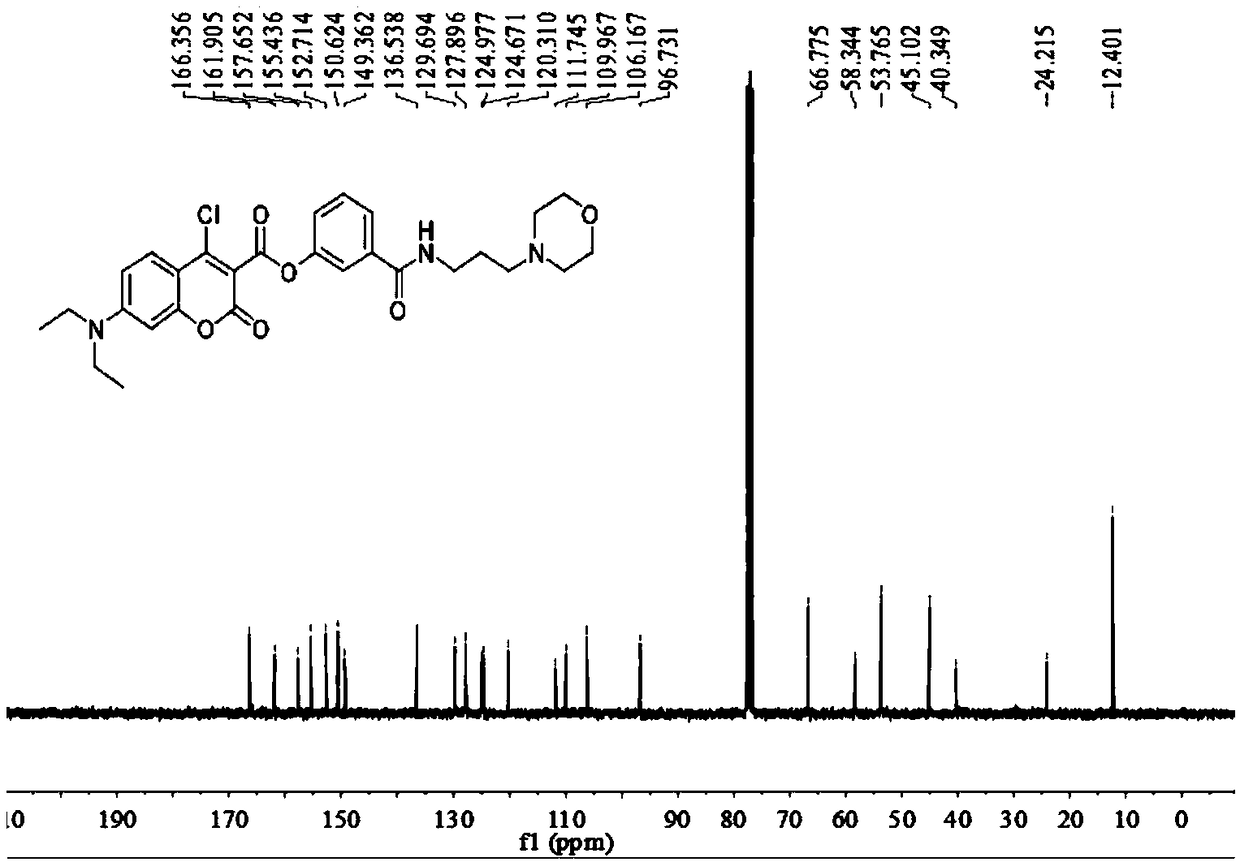
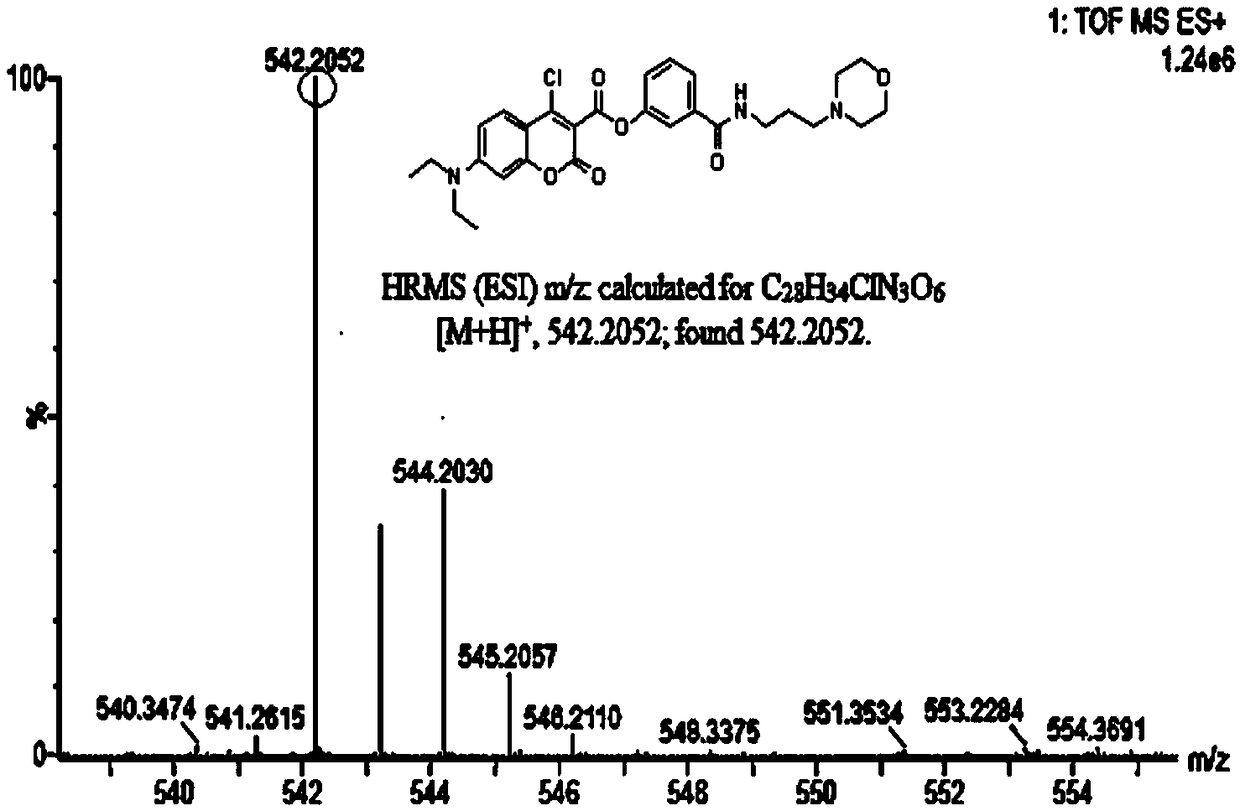
[0059] Add anhydrous acetonitrile (7mL1) to the intermediate (577mg, 1mmol), p-nitrophenol (139mg, 1mmol) and stir to dissolve (specifically add the intermediate and nitrophenol to the reactor first, and then add Anhydrous acetonitrile), then added triethylamine (5 μ L, 1 mmol), under argon protection, refluxed and stirred for 1.5 h, distilled off the solvent under reduced pressure, obtained a light yellow solid through column chromatography, and the light yellow solid was the probe ( probe), yield 577mg, 85%, 1 HNMR spectrum as Figure 4 as shown, 13 CNMR spectrum such as Figure 5 As shown, the mass spectrogram is as Image 6 shown.
Embodiment 3
[0061] Application of Fluorescent Probes
[0062] Dissolve the fluorescent probe in the buffer solution (V DMSO / V PBS =2 / 8, Ph=7.4), and formulated into groups of 1.0×10 - 5 mol / L solution, and then add Cys, GSH, Tyr, Val, Gly, Ala, Asp, Arg, Iso, Lys, Met, His, Phe, Thr, Ser, Pro, Glu to multiple solutions respectively , KCl, CaCl 2 , MgCl 2 , ZnCl 2 , NaCl, H 2 S, and H 2 o 2 , and test the fluorescence intensity of each solution, the test results are as follows Figure 7 with Figure 8 shown;
[0063] Dissolve the fluorescent probe in the buffer solution (V DMSO / V PBS =2 / 8, Ph=7.4), and formulated into groups of 1.0×10 - 5 mol / L solution, and then add different masses of cysteine (Cys) to multiple groups of solutions one by one to prepare a solution with a cysteine concentration of 0-300 μM, and finally test the fluorescence intensity respectively. The results are as follows Figure 9 As shown, the arrow in the figure indicates that the concentration o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com