Chiral guanidine catalysts based on tartaric acid skeleton, preparation method and application thereof
A technology of catalyst and tartaric acid, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, etc., and can solve the problems of limited types of chiral guanidine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
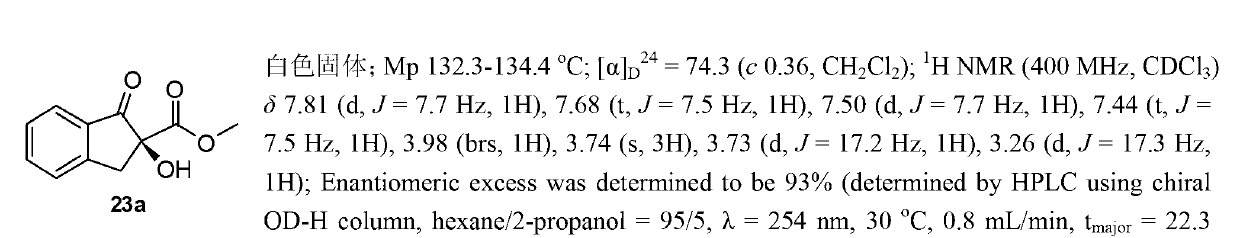
[0077]
[0078] Reagents and conditions: a) 2,2-dimethoxypropane, p-toluenesulfonic acid, benzene, reflux, 12h; b) bromobenzene, magnesium powder, tetrahydrofuran, reflux, 1.5h, yield 91% (two steps reaction); c) thionyl chloride, triethylamine, dichloromethane, reflux, 3h, yield 65%; d) sodium azide, N,N-dimethylformamide, 80°C, 72h, yield 77%; e) lithium aluminum tetrahydrogen, tetrahydrofuran, 0 ℃, 4h, yield 93%; f) carbon disulfide, pyridine, 60 ℃, yield 98%; g) substituted amine, cuprous chloride, potassium carbonate, tetrahydrofuran , 40°C, 4-30h, yield 87-95%.
[0079] 1. Synthesis of G1-1:
[0080] Under nitrogen atmosphere, add anhydrous benzene (148mL), L-diethyl tartrate (12.4g, 60mmol), 2,2-dimethoxypropane (9.4g, 11mL, 90mmol, 1.5 eq.), p-toluenesulfonic acid (0.12g). Warm up to reflux and react for 12h. Cool to room temperature, add NaHCO 3 (1.5 g) continued stirring for 30 min. Add water (100mL), separate the organic phase, extract the aqueous phase tw...
Embodiment 2
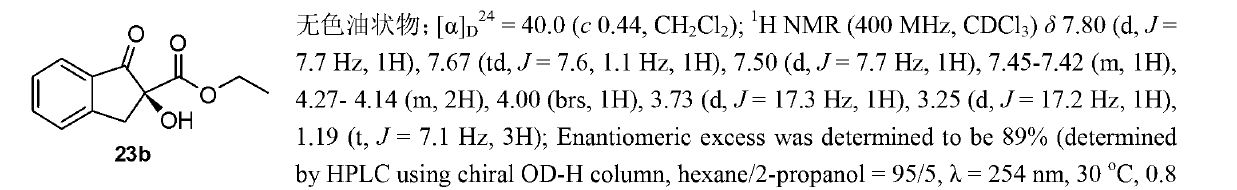
[0096]
[0097] Reagents and conditions: a) 2,2-dimethoxypropane, p-toluenesulfonic acid, benzene, reflux, 12h; b) 4-phenylbromobenzene, magnesium powder, tetrahydrofuran, reflux, 1.5h, yield 95 % (two-step reaction); c) thionyl chloride, triethylamine, dichloromethane, reflux, 3h; d) sodium azide, N,N-dimethylformamide, 80 ° C, 72h, yield 79 % (two-step reaction); e) lithium aluminum tetrahydrogen, tetrahydrofuran, 0 ℃, 4h, yield 84%; f) carbon disulfide, pyridine, 60 ℃, yield 99%; g) substituted amine, cuprous chloride, Potassium carbonate, tetrahydrofuran, 40°C, 15-48h, yield 71-85%.
[0098] 1. Synthesis of G2-1:
[0099] Under nitrogen atmosphere, add anhydrous benzene (148mL), L-diethyl tartrate (4.1g, 20mmol), 2,2-dimethoxypropane (3.1g, 3.7mL, 30mmol, 1.5 eq.), p-toluenesulfonic acid (0.03g). Warm up to reflux and react for 12h. Cool to room temperature, add NaHCO 3 (0.5g) and continue to stir for 30min. Add water (40mL), separate the organic phase, extract t...
Embodiment 3
[0113]
[0114] Reagents and conditions: a) cyclohexanone, p-toluenesulfonic acid, zinc chloride, benzene, reflux, 18h; b) bromobenzene, magnesium powder, tetrahydrofuran, reflux, 1.5h, yield 75% (two-step reaction) ; c) thionyl chloride, triethylamine, dichloromethane, reflux, 3h; d) sodium azide, N,N-dimethylformamide, 80℃, 72h, yield 71% (two-step reaction) ; e) lithium aluminum tetrahydride, tetrahydrofuran, 0 ℃, 4h, yield 84%; f) carbon disulfide, pyridine, 60 ℃, yield 83%; g) p-methylbenzylamine, cuprous chloride, potassium carbonate, Tetrahydrofuran, 40°C, 12h, yield 62%.
[0115] 1. Synthesis of G3-1:
[0116] Under nitrogen atmosphere, add anhydrous benzene (200mL), L-diethyl tartrate (11.5g, 56mmol), cyclohexanone (8.2g, 8.7mL, 84mmol, 1.5eq.), p-toluene Sulfonic acid (0.35g), zinc dichloride (0.35g). The temperature was raised to reflux, and the reaction was carried out for 18h. Cool to room temperature, add NaHCO 3 (1.4g) Stirring was continued for 30min. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com