Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
40 results about "Formate dehydrogenase H" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol
InactiveCN108384765AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesFormate dehydrogenase HGlycol synthesis
The invention discloses an alcohol dehydrogenase mutant and application thereof to synthesis of diaryl chiral alcohol and belongs to the technical field of biological engineering. The alcohol dehydrogenase mutant disclosed by the invention has good catalytic activity and stereoselectivity, and a series of chiral diaryl alcohol with R- and S- configurations can be prepared through efficient catalysis. According to the alcohol dehydrogenase mutant, alcohol dehydrogenase is coupled with glucose dehydrogenase or formate dehydrogenase, and can be used for synthesizing various antihistamine drugs, i.e., a chiral diaryl alcohol intermediate. Compared with an existing report, a method for preparing the diaryl chiral alcohol through asymmetric catalysis of the alcohol dehydrogenase has the advantages of simplicity in operation, high substrate concentration, complete reaction and high product purity and has a very good industrial application prospect.
Owner:JIANGNAN UNIV
Phosphinothricin dehydrogenase mutant, genetically engineered bacteria and one-pot multi-enzyme synchronous directed evolution method
ActiveCN111621482AImprove conversion rateIncreased space-time yieldBacteriaMicroorganism based processesArginineThreonine
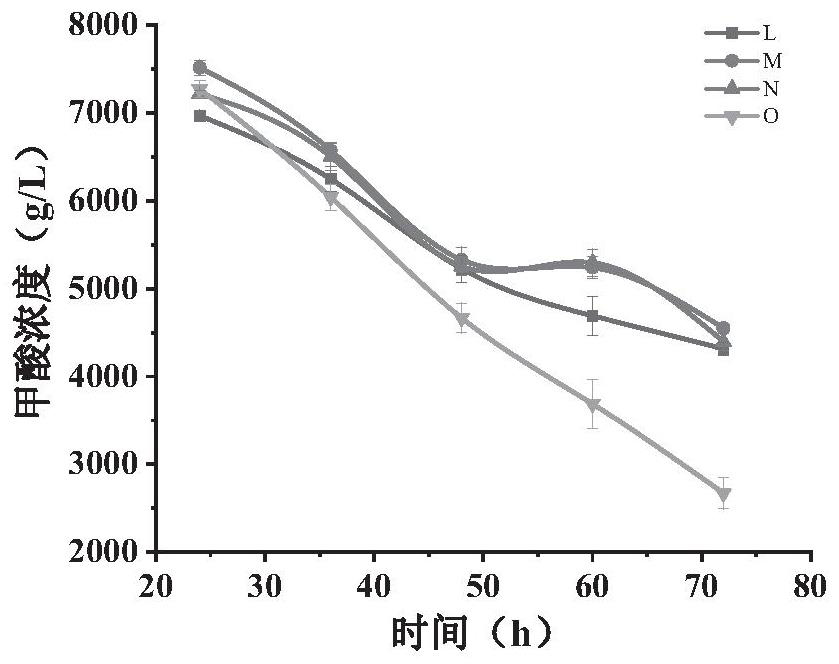
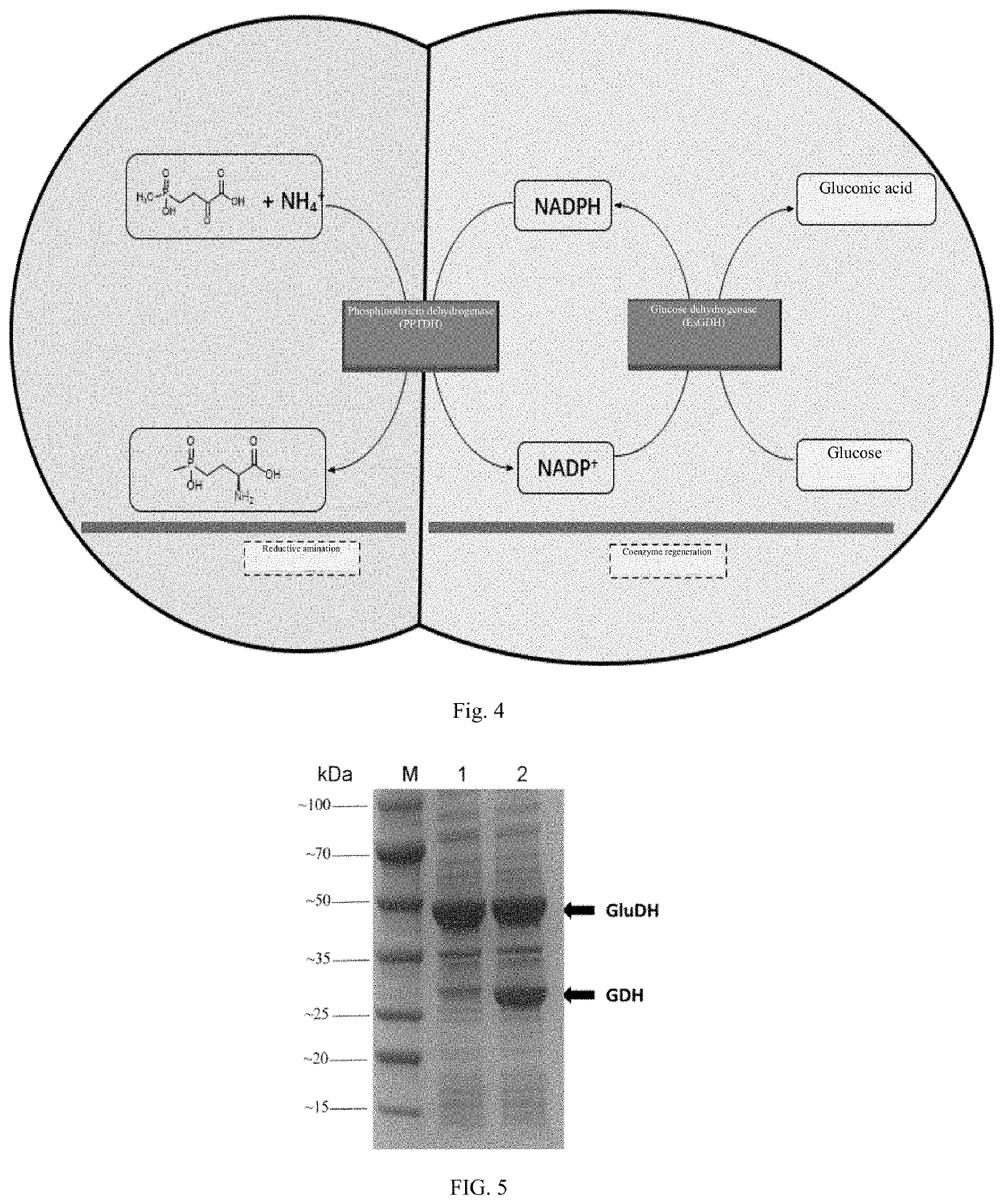
The invention discloses a phosphinothricin dehydrogenase mutant, genetic engineering bacteria and a one-pot multi-enzyme synchronous directed evolution method. The phosphinothricin dehydrogenase mutant is obtained by mutating the 164th amino acid from alanine to glycine, mutating the 205th arginine to lysine and mutating the 332nd threonine to alanine of phosphinothricin dehydrogenase derived fromPseudomonas fluorescens, and an amino acid sequence is as shown in SEQ ID No.1. The genetically engineered bacteria are obtained by introducing a gene of the phosphinothricin dehydrogenase mutant into a host cell. An encoding gene of glucose dehydrogenase or an encoding gene of formate dehydrogenase can also be introduced into the host cell to perform simultaneous directed evolution to overexpress the double genes. The one-pot multi-enzyme synchronous directed evolution method of the invention can screen out the genetically engineered bacteria with greatly improved activity. Compared with catalytic processes such as transaminase, the L-PPT preparation method of the invention has a relatively simple process, a high conversion rate of raw materials, a conversion rate of up to 100%, and highstereo-selectivity.
Owner:ZHEJIANG UNIV OF TECH
Enhancement of Microbial Ethanol Production
InactiveUS20090226992A1Easy to transformEfficient fermentationSugar derivativesBacteriaLactate dehydrogenaseDNA construct
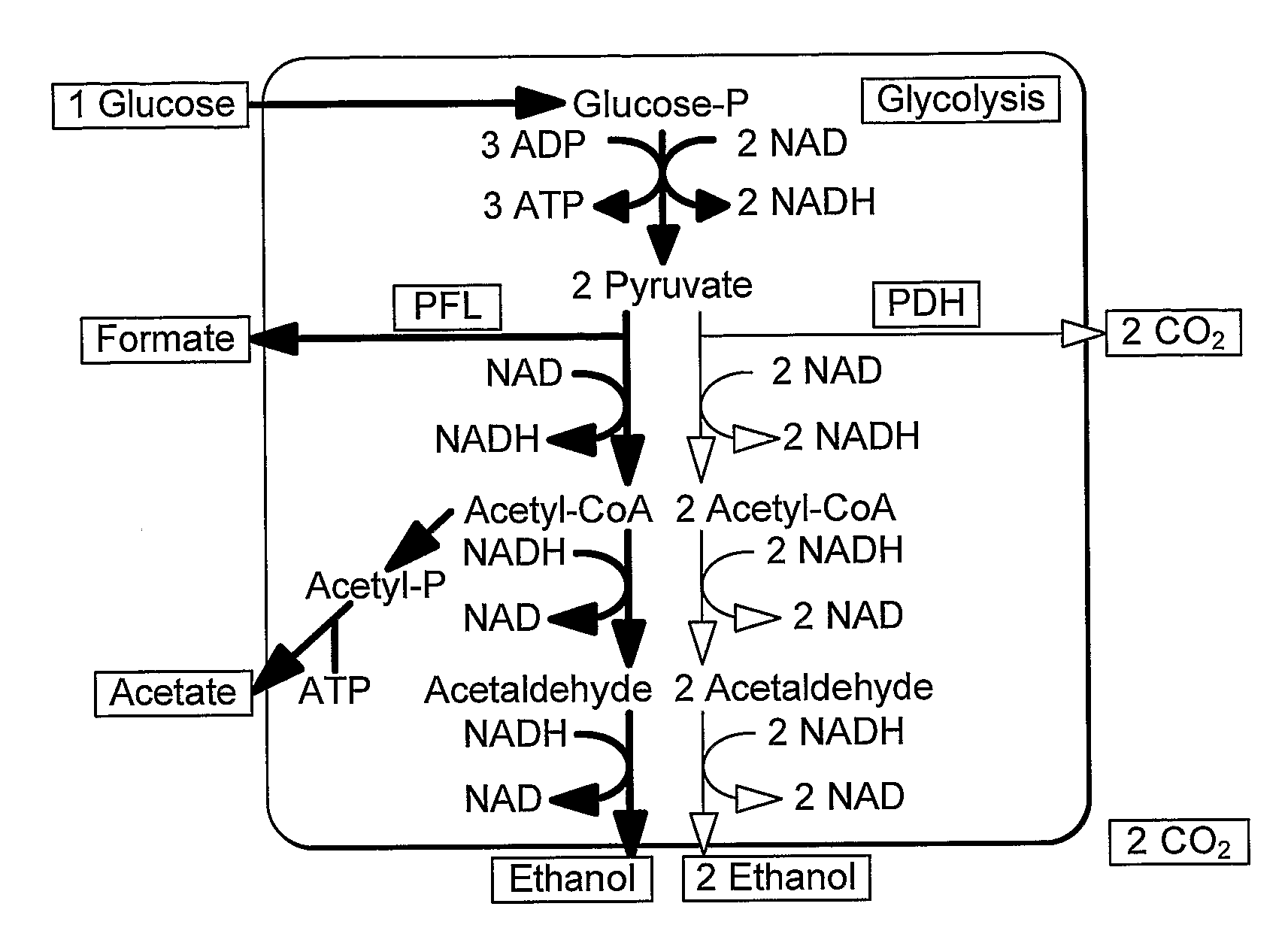
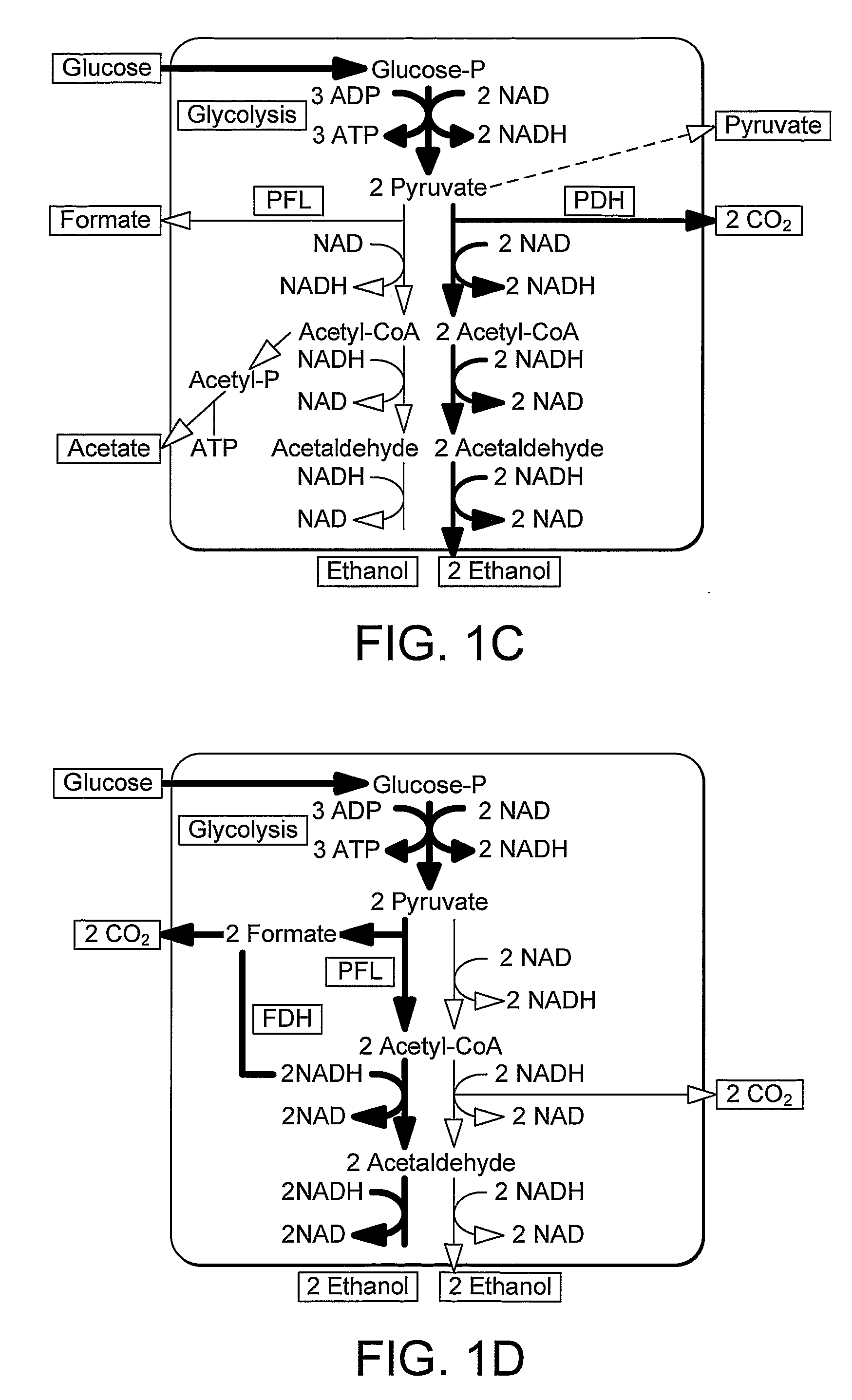
A thermophilic microorganism lacks lactate dehydrogenase activity and preferably contains an active pyruvate formate lyase pathway. The thermophilic microorganism contains a gene encoding an NAD-linked formate dehydrogenase. The gene encoding an NAD-linked formate dehydrogenase is preferably a codon optimised version of the gene encoding a thermostable NAD-linked formate dehydrogenase. DNA constructs allow stable expression of the gene encoding an NAD-linked formate dehydrogenase in the thermophilic microorganism. The DNA constructs are based upon use of an insertion sequence to achieve stable expression or recombination to insert the gene encoding an NAD-linked formate dehydrogenase into the lactate dehydrogenase gene, thus achieving gene knockout and new functionality in a single step. The microorganisms are useful in fermentation of sugars to produce ethanol.
Owner:BIOCONVERSION TECH LTD
Preparation method and application of sludge petroleum degrading complex enzyme
ActiveCN108486006AEfficient Pollution Treatment CapabilityReduce manufacturing costBacteriaWater contaminantsHigh concentrationSludge
The invention relates to a preparation method and application of a sludge petroleum degrading complex enzyme. The preparation method comprises the following steps: (1), performing cell disruption on acinetobacter calcoaceticus, centrifuging, and taking a supernatant to obtain a petroleum degrading enzyme solution 21#; (2), mixing the petroleum degrading enzyme solution 21# and formate dehydrogenase to obtain the sludge petroleum degrading complex enzyme. For the first time, after a petroleum-degrading enzyme system derived from acinetobacter calcoaceticus is mixed with the formate dehydrogenase in proportion, the sludge petroleum degrading complex enzyme can be applied to degradation and repair of petroleum in high-concentration petroleum-contaminated sludge, so that immobilization of adsorbents such as diatomite is not required, and the petroleum in the sludge can be reduced in a short time. The sludge petroleum degrading complex enzyme has high-efficiency petroleum contamination treatment capacity, has low production cost, and has a wide application prospect.
Owner:ECOLOGY INST SHANDONG ACAD OF SCI
Mycobacterium vaccae formic dehydrogenase mutant and its use
The object of the present invention is to provide polypeptides that can maintain strong formate dehydrogenase activity in the presence of organic solvents, and their applications. By replacing cysteine at positions 146 and / or 256 in the amino acid sequence of formate dehydrogenase derived from Mycobacterium vaccae through site-directed mutagenesis, a formate dehydrogenase mutant polypeptide is constructed, which is resistant to organic solvents. sex. This type of polypeptide has strong formate dehydrogenase activity in the presence of organic solvents. Such mutants can use ketones as raw materials to produce alcohols, etc.
Owner:DAICEL CHEM IND LTD
Formate dehydrogenase tolerant to halogen compounds and process for producing the same
InactiveUS7432095B2Decrease productivityImprove efficiencySugar derivativesBacteriaA-DNARecombinant DNA
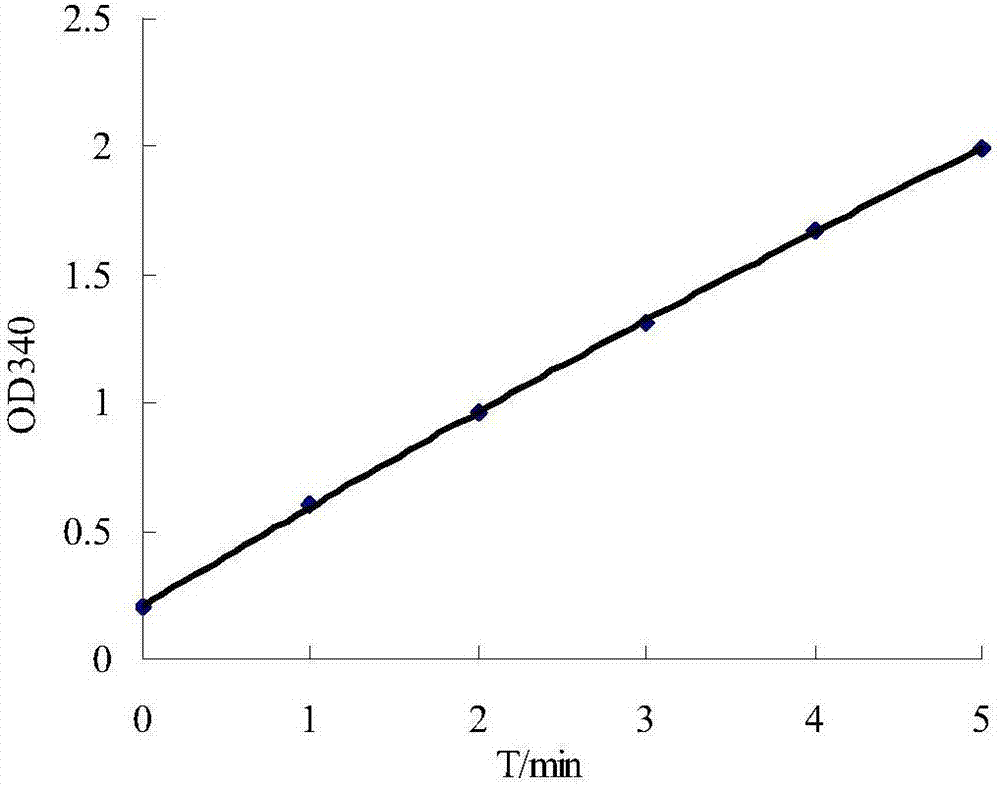
The invention provides a formate dehydrogenase having high specific activity, small Km values for formate and NAD, broad temperature and pH ranges of stability, broad temperature and pH ranges for action, and capable of regenerating a coenzyme with good efficiency in case of being present in an enzymatic reduction reaction system without inactivated.The invention provides the enzyme mentioned above which has characteristics suited for industrial application by screening for soil formate dehydrogenase-producing microorganisms. The invention further provides a DNA containing the gene coding for the enzyme of the invention, a recombinant DNA constructed using a vector, and a transformant obtained by using this plasmid. The invention further provides a process for producing the formate dehydrogenase of the invention by using any microorganism of the genus Thiobacillus or a transformant constructed by using a formate dehydrogenase gene derived from above strain of microorganism, and a process for regenerating a coenzyme by using said enzyme in an enzymatic reduction system.
Owner:KANEKA CORP
Alcohol dehydrogenase mutant, and application thereof in synthesis of bisaryl chiral alcohols
InactiveCN108504641AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesAlcoholFormate dehydrogenase
The invention discloses an alcohol dehydrogenase mutant, and an application thereof in the synthesis of bisaryl chiral alcohols, and belongs to the technical field of bioengineering. The alcohol dehydrogenase mutant has excellent catalytic activity and stereoselectivity, and achieve efficiently catalyzed preparation of a series of chiral bisaryl alcohols with R- and S- configurations. Alcohol dehydrogenase is coupled with glucose dehydrogenase or formate dehydrogenase in order to synthesize multiple antihistamine chiral bisaryl alcohol intermediates. Compared with existing reports, the methodfor preparing the bisaryl chiral alcohols by asymmetric catalytic reduction of the alcohol dehydrogenase has the advantages of simplicity in operation, high substrate concentration, completeness in reaction, high product purity and great industrial application prospect.
Owner:JIANGNAN UNIV
Construction method and application of formate dehydrogenase engineering strain
ActiveCN107299074AIncrease enzyme activityStrong industrial applicabilityBacteriaMicroorganism based processesEscherichia coliCompetent cell
The invention relates to a construction method and application of a formate dehydrogenase engineering strain. The construction method comprises the following steps: (1) extracting candida boidinii genome DNA as a template; (2) with the genome DNA as the template, performing PCR amplification to obtain an fdh gene segment; (3) performing double digestion on the fdh gene segment and connecting to a plasmid pET28a(+) to obtain a plasmid pET28a(+)-fdh; (4) transforming the plasmid pET28a(+)-fdh into an escherichia coli BL21(DE3) competent cell to obtain a recombinant strain BL21(DE3)-fdh for expressing fdh. According to the construction method provided by the invention, a primer pair is degenerate primers designed according to the fdh sequence of multiple strains of candida boidinii registered in NCBI (National Center of Biotechnology Information); An fdh gene can be amplified by taking the genome DNA of most candida boidinii strains as the template while the influence of individual base difference in the gene sequence is avoided.
Owner:ECOLOGY INST SHANDONG ACAD OF SCI
Formate dehydrogenase mutant with improved substrate affinity and coenzyme affinity
ActiveCN110408604AHigh catalytic efficiencyIncrease lifting powerOxidoreductasesFermentationArginineFormate dehydrogenase H
The invention relates to an NADP+ dependent formate dehydrogenase mutant with significantly improved substrate affinity and coenzyme affinity and a coding nucleic acid of the mutant, and a recombinantexpression vector and a recombinant expression transformant containing a nucleic acid sequence. The formate dehydrogenase mutant is a derived protein constituted by a new amino acid sequence formed by substituting one or more amino acid residues of 30th arginine, 124th isoleucine, 146th glycine, 216th glycine, 261st proline, 262nd serine, 287th alanine, 361st arginine or 381st glutamine in an amino acid sequence shown in SEQ ID No.2 with other amino acid residues; the substrate affinity and the coenzyme affinity of the derived protein are higher than those of formate dehydrogenase constitutedby the amino acid sequence shown in SEQ ID No.2. Compared with the prior art, the mutant has the advantages of high substrate affinity and coenzyme affinity, and has a good application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Genetically engineered bacterium for producing free fatty acid under assistance of formate
The invention relates to a genetically engineered bacterium for producing free fatty acid under the assistance of formate, the genetically engineered bacterium is recombinant saccharomyces cerevisiae containing a formate dehydrogenase gene FDH, a 1, 5-diphosphate carboxylase / oxygenase gene RuBisCO and a ribulose phosphate kinase gene PRK, and the genetically engineered bacterium is subjected to chassis microbial modification of knockout of related genes of an FFAs catabolism pathway. The genetically engineered bacterium is safe and non-toxic, can enable microorganisms to produce FFAs by utilizing carbon dioxide and a derivative formic acid thereof through fermentation, and is environment-friendly, relatively low in production cost and wide in application prospect.
Owner:BEIJING UNIV OF CHEM TECH
Recombinant microorganism and method for production of formic acid by using same
ActiveUS20200095620A1Promote disseminationEasy to transportBacteriaOxidoreductasesMicroorganismMicrobiology
The present invention relates to a recombinant microorganism for producing formic acid, which has a formate dehydrogenase 1 alpha subunit (FDH1α)-encoding endogenous gene deleted therefrom and an FDH1-encoding exogenous gene introduced thereinto, and a method for production of formic acid by using the microorganism.
Owner:UNIST ULSAN NAT INST OF SCI & TECH
Method for measuring activity of formate dehydrogenase in plant leaves and evaluating activity of formate dehydrogenase
PendingCN113817801AReduce contentChemical analysis using titrationMicrobiological testing/measurementBiotechnologyMicrobiology
The invention discloses a method for measuring the activity of formate dehydrogenase in plant leaves and evaluating the activity of formate dehydrogenase. The activity of formate dehydrogenase in the leaf extracting solution is eliminated in a high-temperature treatment manner, and according to the chemical property of formic acid, a potentiometric titration method is adopted to quantitatively evaluate the activity of formate dehydrogenase in plant leaves through measuring the reduction value of the formaldehyde content in the plant fresh extracting solution and the inactivated extracting solution and the acidity change difference in the plant fresh extracting solution and the inactivated extracting solution before and after the formaldehyde is added. Experimental results show that when the ratio of the plant mass to the extracting solution is 1: 4 and the formaldehyde concentration is 350 mg.L <-1>, the activity of the formate dehydrogenase is sequentially as follows: the activity of the scindapsus aureus is greater than that of peony chlorophytum comosum, and the activity of the peony chlorophytum comosum is greater than that of aloe; when the formaldehyde concentration is 500-750 mg.L <-1>, the activity of the formate dehydrogenase is as follows: the activity of the peony chlorophytum comosum is greater than that of the scindapsus aureus, and the activity of the formate dehydrogenase is not detected in the aloe leaf extracting solution.
Owner:XINJIANG UNIVERSITY
Alcohol Dehydrogenase Mutant and Application thereof in Synthesis of Diaryl Chiral Alcohols
ActiveUS20190345455A1High activityHigh stereoselectivityOxidoreductasesFermentationFormate dehydrogenaseAlcohol dehydrogenase
The present disclosure discloses an alcohol dehydrogenase mutant and application thereof in synthesis of diaryl chiral alcohols, and belongs to the technical field of bioengineering. The alcohol dehydrogenase mutant of the present disclosure has excellent catalytic activity and stereoselectivity, and may efficiently catalyze the preparation of a series of chiral diaryl alcohols in R- and S-configurations. By coupling alcohol dehydrogenase of the present disclosure to glucose dehydrogenase or formate dehydrogenase, the synthesis of chiral diaryl alcohol intermediates of various antihistamines may be achieved. Compared with the prior art, a method for preparing diaryl chiral alcohols through asymmetric catalytic reduction using the alcohol dehydrogenase of the present disclosure has the advantages of simple and convenient operation, high substrate concentration, complete reaction and high product purity, and has great industrial application prospects.
Owner:JIANGNAN UNIV
Genetic engineering strain for efficiently producing 1,3-propanediol, construction method for genetic engineering strain and application of genetic engineering strain
ActiveCN111996157AHigh glycerol toleranceImprove induction abilityBacteriaMicroorganism based processesNADH regenerationBatch fermentation
The invention relates to a genetic engineering strain for efficiently producing 1,3-propanediol, a construction method for the genetic engineering strain and application of the genetic engineering strain. According to the genetic engineering strain, the construction method therefor and the application of the genetic engineering strain, the enzyme activity of 1,3-propanediol oxidoreductase is improved through strengthening expression of the 1,3-propanediol oxidoreductase in Klebsiella pneumoniae, meanwhile, a NADH regeneration way is constructed, thus, the accumulation of 3-hydroxypropanal is lowered, the conversion of the 1,3-propanediol from the 3-hydroxypropanal is strengthened, and thus, the yield of the 1,3-propanediol is increased. The enzyme activity of the genetic engineering strain, i.e., the 1,3-propanediol oxidoreductase, constructed by the method is 3.25 to 3.5 times that of a starting strain; and the enzyme activity of formate dehydrogenase is 0.414U / mg to 0.45U / mg. Throughcarrying out fed-batch fermentation for 29 hours by employing the genetic engineering strain constructed by the method, the yield of the 1,3-propanediol is increased by 43.89%-97.93% compared with that of the starting strain; and due to a dual-gene synergistic effect, the output and yield of the 1,3-propanediol are increased remarkably.
Owner:QILU UNIV OF TECH
Formate dehydrogenase mutant and application thereof
ActiveCN114752572AHigh reductase activityEfficient reductionBacteriaMicroorganism based processesFormate dehydrogenase HAcyl CoA dehydrogenase
The invention discloses a formate dehydrogenase mutant and application thereof. The formate dehydrogenase mutant comprises mutation of at least one amino acid in the 54th site, the 121st site and the 228th site of an amino acid sequence as shown in SEQ ID No.1. Compared with conventional wild type formate dehydrogenase, the formate dehydrogenase provided by the invention has higher reductase activity on NMN, the enzyme activity of the formate dehydrogenase reaches about 80-886 times that of the wild type, the formate dehydrogenase can efficiently reduce the NMN into NMNH, and the formate dehydrogenase can be used in the form of crude enzyme without purification or can be used only after partial purification due to the high catalytic activity. Therefore, the production cost can be greatly reduced, and the method is suitable for large-scale industrial production and has better market competitiveness.
Owner:深圳希吉亚生物技术有限公司
Glufosinate-ammonium dehydrogenase mutant, engineering bacterium, immobilized cell and application
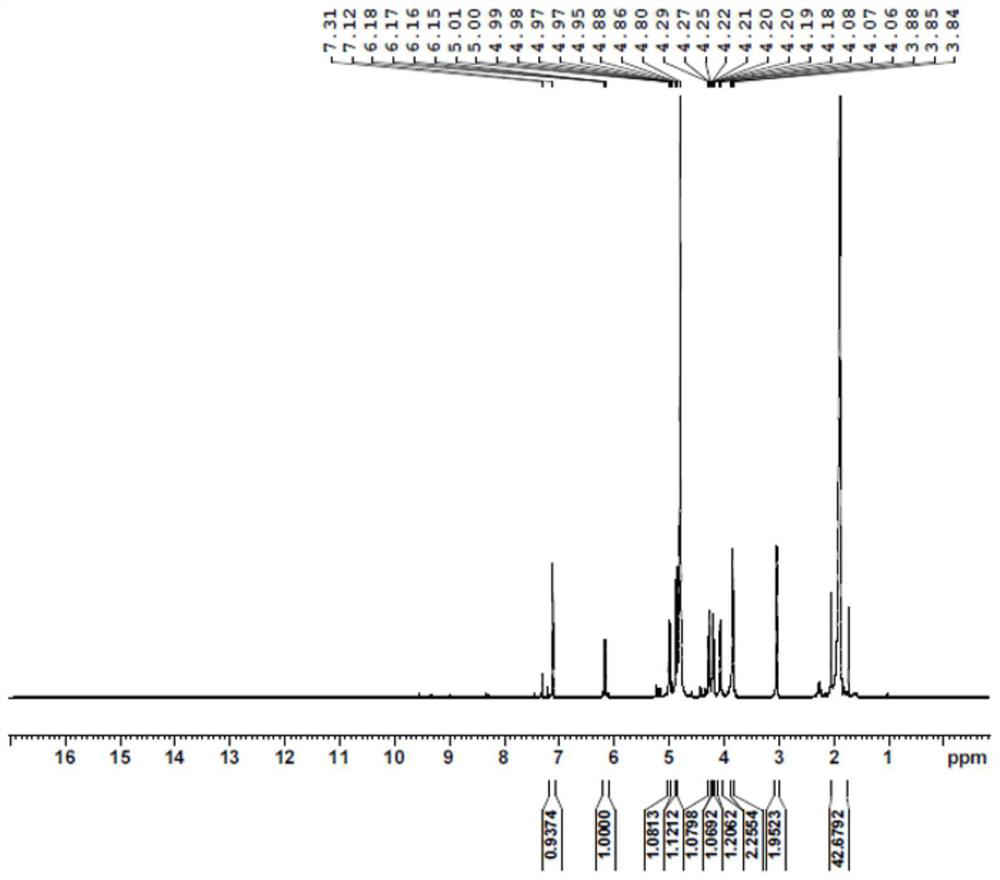
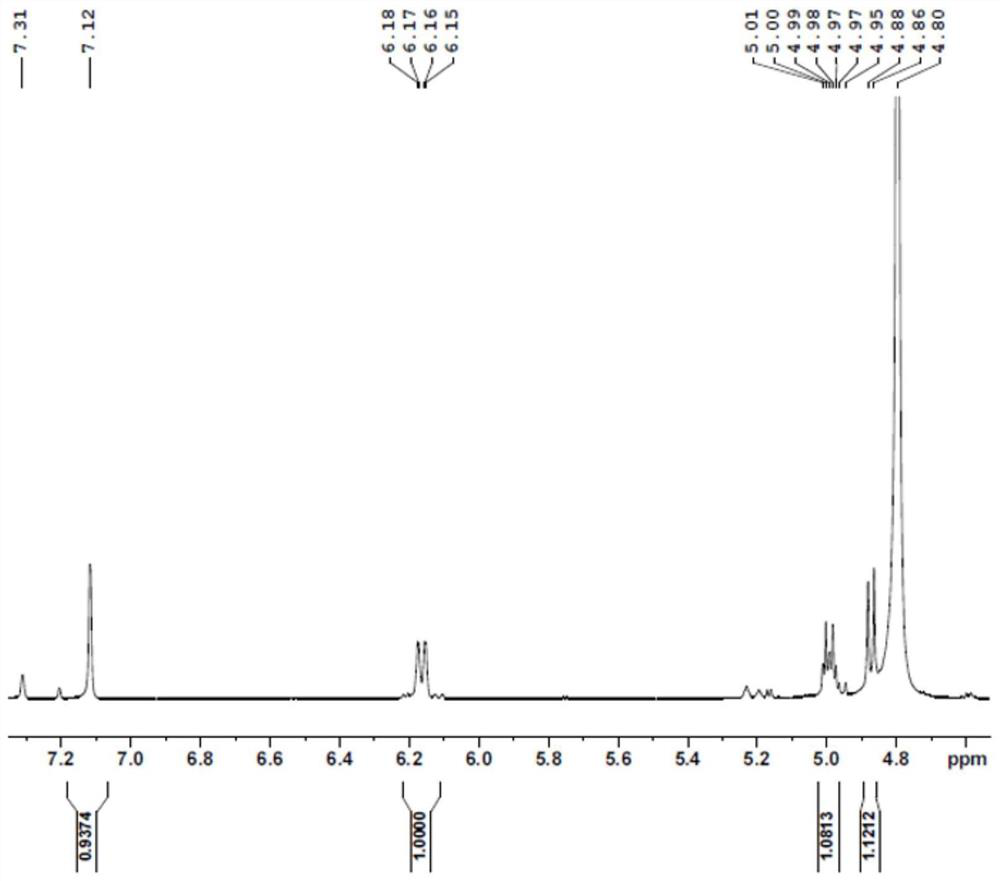
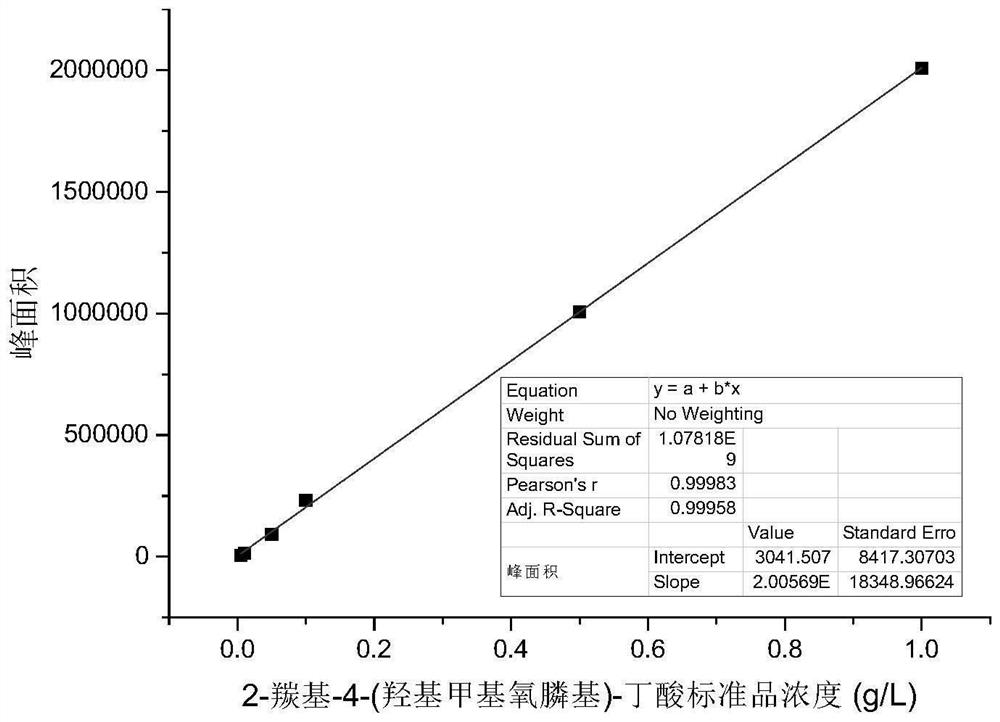
PendingCN114350631AImprove thermal stabilityLow costBacteriaMicroorganism based processesAcyl groupFormate dehydrogenase H
The invention discloses a glufosinate-ammonium dehydrogenase mutant, an engineering bacterium, an immobilized cell and application. The mutant is obtained by carrying out single mutation or multiple mutations on the 145th site, the 384th site, the 348th site, the 292th site, the 202 site, the 70th site and the 78th site of a glufosinate-ammonium dehydrogenase amino acid sequence shown in SEQ ID No.2. According to the immobilized cell of the co-expression engineering bacterium of the glufosinate-ammonium dehydrogenase mutant and the formate dehydrogenase, the L-glufosinate-ammonium is obtained through catalytic reduction by using a 2-carbonyl-4-(hydroxymethylphosphonyl)-butyric acid substrate, the asymmetric synthesis of the L-glufosinate-ammonium is realized, an expensive chemical resolution reagent is not needed, the thermal stability and the operation stability of the L-glufosinate-ammonium are improved, and the repeated use is realized. The enzyme activity of the immobilized cells is 162.5 U / g, the enzyme activity recovery rate is 78.1%, and the immobilized cells can be recovered through filtration and can be repeatedly used for more than 20 batches to keep 100% conversion rate, so that the production cost is greatly reduced.
Owner:ZHEJIANG UNIV OF TECH
Alcohol dehydrogenase and its coding gene and its application in catalytic synthesis of (r)-phenylethylene glycol
ActiveCN111944774BAchieve in situ regenerationReduce inhibitionBacteriaMicroorganism based processesPhosphatePhenyl glycol
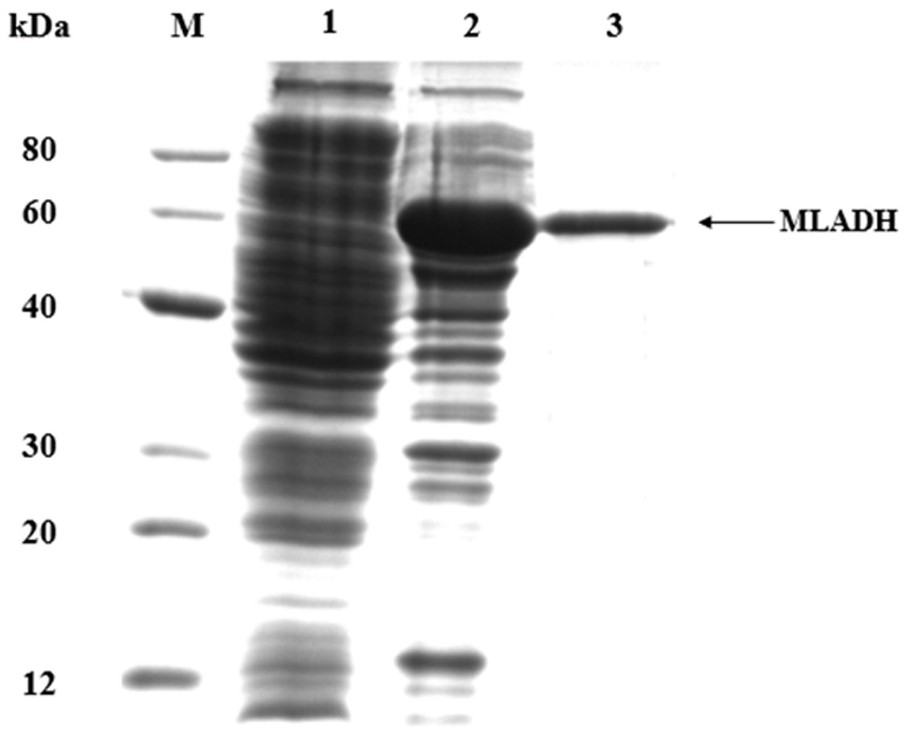
The invention provides a kind of alcohol dehydrogenase and its encoding gene and its catalytic synthesis ( R )-phenylethylene glycol, the amino acid sequence of the alcohol dehydrogenase is shown in sequence 1 in the sequence listing, and its gene sequence is shown in sequence 2 in the sequence listing. alcohol dehydrogenase gene mladh In catalytic synthesis ( R )‑Phenylglycol. Alcohol dehydrogenase MLADH and formate dehydrogenase FDH were used to construct a dual-enzyme coupled catalytic system. In the system, the enzyme activity ratio of alcohol dehydrogenase MLADH and formate dehydrogenase FDH was 1:5-10. The invention constructs a double-enzyme coupling system of alcohol dehydrogenase MLADH and formate dehydrogenase FDH, and realizes the in-situ regeneration of coenzyme NADH. In the present invention, soybean oil is introduced into the system using phosphate buffer salt as the water phase as the organic phase, which reduces the inhibition of the catalytic reaction by the substrate and the product, promotes the efficiency of the catalytic conversion, and the conversion rate reaches 99%. e.e. The value is above 99%.
Owner:HEBEI UNIVERSITY
Phosphinothricin dehydrogenase mutant, genetically engineered bacterium and one-pot multi-enzyme synchronous directed evolution method
PendingUS20220307061A1Efficient processingNumber highBacteriaMicroorganism based processesArginineThreonine
Disclosed are a phosphinothricin dehydrogenase mutant, a recombinant bacterium and a one-pot multi-enzyme synchronous directed evolution method. The phosphinothricin dehydrogenase mutant, with an amino acid sequence as shown in SEQ ID No.1, is obtained by mutating alanine at position 164 to glycine, arginine at position 205 to lysine, and threonine at position 332 to alanine in a phosphinothricin dehydrogenase derived from Pseudomonas fluorescens. The recombinant bacterium is obtained by introducing a gene encoding the phosphinothricin dehydrogenase mutant into a host cell. The host cell can also incorporate a gene encoding a glucose dehydrogenase or a gene encoding a formate dehydrogenase to undergo synchronous directed evolution to achieve double gene overexpression. The one-pot multi-enzyme synchronous directed evolution method of the present invention can screen recombinant bacteria with greatly improved activity. Compared with other catalysis processes such as the transaminase method, the method for preparing L-PPT of the present invention features relatively simple process, high conversion of raw materials of up to 100%, and high stereo selectivity.
Owner:ZHEJIANG UNIV OF TECH
A kind of genetic engineering bacteria for efficient production of 1,3-propanediol and its construction method and application
ActiveCN111996157BHigh glycerol toleranceImprove induction abilityBacteriaMicroorganism based processesNADH regenerationBatch fermentation
The invention relates to a genetically engineered bacterium capable of efficiently producing 1,3-propanediol and a construction method and application thereof. The present invention strengthens the expression of 1,3-propanediol oxidoreductase in Klebsiella pneumoniae to enhance the enzymatic activity of 1,3-propanediol oxidoreductase, and simultaneously constructs a NADH regeneration pathway to reduce the accumulation of 3-hydroxypropionaldehyde, Enhances the conversion of 3-hydroxypropionaldehyde to 1,3-propanediol, thereby increasing the yield of 1,3-propanediol. The enzymatic activity of the 1,3-propanediol oxidoreductase of the genetically engineered bacteria constructed by the present invention is 3.25-3.5 times that of the starting strain; the enzymatic activity of the formate dehydrogenase is 0.414-0.45 U / mg. Using the genetically engineered bacteria constructed by the present invention to carry out fed-batch fermentation for 29 hours, the yield of 1,3-propanediol is increased by 43.89%-97.93% compared with the starting strain, and the synergistic effect of the double genes significantly increases the yield of 1,3-propanediol and yield.
Owner:QILU UNIV OF TECH
A method for enzymatically synthesizing l-2-aminobutyric acid
ActiveCN107012178BReduce lossesReduced activityFermentationFormate dehydrogenase HAlanine dehydrogenase
The invention discloses a method for synthesizing L-2-aminobutyric acid by an enzymatic method. According to the method, 2-ketobutyric acid is catalyzed by the aid of alanine dehydrogenase and formate dehydrogenase to produce the L-2-aminobutyric acid. The method particularly includes the steps: uniformly mixing 0.5-1.5mol of 2-ketobutyric acid, 0.5-1.5mol of ammonium formate, o-0.5g / L of NAD (nicotinamide adenine dinucleotide) and 20g / L of formate dehydrogenase and alanine dehydrogenase co-expression wet cells (or 10-30g / L of formate dehydrogenase wet cells, 10-30g / L of alanine dehydrogenase wet cells) in a rector; adjusting a pH (potential of hydrogen) value to be 7.0-9.0; performing catalytic reaction for 16-20h at the temperature ranging from 30 DEG C to 37 DEG C to obtain the L-2-aminobutyric acid. The method is rapid in reaction, reverse oxidation deamination activity is low, and product loss is greatly reduced.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
Carbonyl reductase mutant as well as construction method and application thereof
ActiveCN113462666AIncrease enzyme activitySolve concentrationBacteriaMicroorganism based processesGlycineCarbonyl Reductase
The invention relates to the field of biochemical engineering, and discloses a carbonyl reductase mutant as well as a construction method and application thereof.The 143rd leucine in an amino acid sequence shown in SEQ ID NO.2 is mutated into alanine, the 136th leucine is mutated into alanine, and 135th glycine is mutated into isoleucine, so that the enzyme activity of the carbonyl reductase mutant is improved by 8 times; and the e.e. Value is increased to 99%, coenzyme circulation is successfully realized by coupling with formate dehydrogenase, and finally, the conversion rate and the e.e. Value of a 450 g / L substrate within 12 hours can be over 99% when the final concentration of thalli is 20 g (dcw) / L, so that the problems of low substrate concentration, low conversion rate, low yield, low e.e. Value, relatively high production cost and the like are effectively solved.
Owner:杭州文德阶生物科技有限公司
Mutants of formate dehydrogenase with improved substrate affinity and coenzyme affinity
ActiveCN110408604BHigh catalytic efficiencyIncrease lifting powerOxidoreductasesGenetic engineeringArginineFormate dehydrogenase H
The invention relates to an NADP+ dependent formate dehydrogenase mutant with significantly improved substrate affinity and coenzyme affinity and a coding nucleic acid of the mutant, and a recombinantexpression vector and a recombinant expression transformant containing a nucleic acid sequence. The formate dehydrogenase mutant is a derived protein constituted by a new amino acid sequence formed by substituting one or more amino acid residues of 30th arginine, 124th isoleucine, 146th glycine, 216th glycine, 261st proline, 262nd serine, 287th alanine, 361st arginine or 381st glutamine in an amino acid sequence shown in SEQ ID No.2 with other amino acid residues; the substrate affinity and the coenzyme affinity of the derived protein are higher than those of formate dehydrogenase constitutedby the amino acid sequence shown in SEQ ID No.2. Compared with the prior art, the mutant has the advantages of high substrate affinity and coenzyme affinity, and has a good application prospect.
Owner:EAST CHINA UNIV OF SCI & TECH +1
Method for preparing chiral 3R, 5S-dihydroxyl compound by nonaqueous phase
ActiveCN101880694BHigh substrate concentrationPractical application value is greatFermentationBiotechnologyFormate dehydrogenase H
A process for preparing ethyl (3R, 5S)-dihydroxy-6-benzyloxy hexanoate via stereoselective microbial reduction is provided, which includes that ethyl 3,5-diketo-6-benzyloxy hexanoate is used as a substrate and a diketoreductase is used as biocatalyst. Specifically, the process includes these following steps: (1) the substrate, diketoreductase and cofactor regeneration system mediated by formate dehydrogenase are added to the reaction liquid and conducted to shake and react for at least one hour, and the reaction liquid is the mixture of organic solvent and buffer solution; (2) the product of step (1) is separated and purified to obtain the single enantiomer of (3R, 5S)-dihydroxy compound.
Owner:SICHUAN TONGRENTAI PHARMA
Method for reducing NAD analogue by using formic acid
The invention discloses a method for reducing an NAD analogue by using formic acid and application of the method. In the method, a reducing agent is a formic acid compound, a catalyst is formate dehydrogenase capable of utilizing the formic acid compound, and while the formate dehydrogenase oxidizes the formic acid compound, the NAD analogue is converted into a reduction state. The method can be used for producing a reduction-state NAD analogue or a deuterated reduction-state analogue. The reducing power can also be provided for the enzymatic reaction consuming the reduction-state NAD analogue; the reduction-state NAD analogue can be used as a coenzyme to be applied to enzyme-catalyzed reduction reactions of malic enzyme ME-L310R / Q401C, D-lactic dehydrogenase DLDH-V152R, saccharomyces cerevisiae alcohol dehydrogenase and the like, and wide application of the NAD analogue is facilitated. The reduction method of the NAD analogue can be used for regenerating the reduced NAD analogue for preparing malic acid or lactic acid under a mild condition; and can also be used as a redox force to regulate the metabolic intensity of malic acid or lactic acid in microorganisms.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Recombinant microorganism and method for production of formic acid by using same
ActiveUS11230703B2Promote disseminationEfficient productionBacteriaMicroorganism based processesMicroorganismMicrobiology
Owner:UNIST (ULSAN NAT INST OF SCI & TECH)
Preparation method of reduced nicotinamide adenine dinucleotide
PendingCN114107412AGood for volatilizationFacilitate post-processing purificationEnzyme stabilisationOxidoreductasesFormate dehydrogenase HHigh activity
The invention relates to the technical field of industrial catalysis, in particular to a preparation method of reduced nicotinamide adenine dinucleotide, which comprises the following steps: 1) screening the activity of formate dehydrogenase at high temperature; 2) screening the activity of formate dehydrogenase under an acidic condition; (3) according to the results of the step (1) and the step (2), formate dehydrogenase with relatively high activity under a high-temperature condition and an acidic condition is selected for later use; respectively weighing ammonium formate and NAD (Nicotinamide Adenine Dinucleotide) into a flask, adding pure water, stirring and dissolving in a water bath, and regulating the pH to be acidic by using sodium hydroxide; finally, formate dehydrogenase obtained through screening is added for reaction, sodium hydroxide is used for maintaining the pH value to be acidic, and the reduced nicotinamide adenine dinucleotide is prepared. According to the method, the reaction can be completed in a short time, so that hydrolysis of a product under an acidic condition is avoided, and carbonate generated under an alkaline condition is also avoided; after the reaction, no large amount of carbonate exists, and the purification cost is low.
Owner:NANJING NUOYUN BIOLOGICAL TECH CO LTD
Genetically modified yeast with increased succinic acid production
Improved yeast cell with increased succinic acid production based on yield and titer. Increased activity of one or more enzymes involved in the pentose phosphate pathway, reducing flux through phosphoglucose isomerase, increasing flux to cytoplasmic acetyl-CoA, installation of a malic enzyme, and / or installation of a formate dehydrogenase leads to increased production of succinic acid.
Owner:PTT GLOBAL CHEMICAL PUBLIC COMPANY LIMITED
A Novel Method for Simultaneous Biosynthesis of 1,3-Propanediol and Acetoin
ActiveCN107099558BImprove the efficiency of separation and purificationBacteriaMicroorganism based processesGlycerolFormate dehydrogenase H
Owner:EAST CHINA UNIV OF SCI & TECH
Efficient preparation method of D-pantoic acid
InactiveCN114657217AProtection securityPrevent leakageOrganic chemistryFermentationFormate dehydrogenase HValine
The invention relates to an efficient preparation method of D-pantoic acid, which specifically comprises the following steps: (1) valine, methanol and compound enzyme are mixed with water, and the compound enzyme comprises L-amino acid deaminase, methanol dehydrogenase, aldolase, formate dehydrogenase and ketopantoic acid reductase; (2) ammonium formate is added, and D-pantoic acid is prepared through an enzyme catalytic reaction under the conditions that the temperature is 25-55 DEG C and the pH value is 4-7; wherein the molar ratio of the valine to the methanol is (0.9 to 1.1): (0.9 to 1.1). According to the method, valine and methanol are used as substrates for fermentation and conversion to generate D-pantoic acid, the raw materials are cheap and easy to obtain, and the reaction cost is low.
Owner:ANHUI HUAHENG BIOTECH +1
Recombinant engineering bacterium and application thereof in efficient conversion of L-pantoic acid lactone
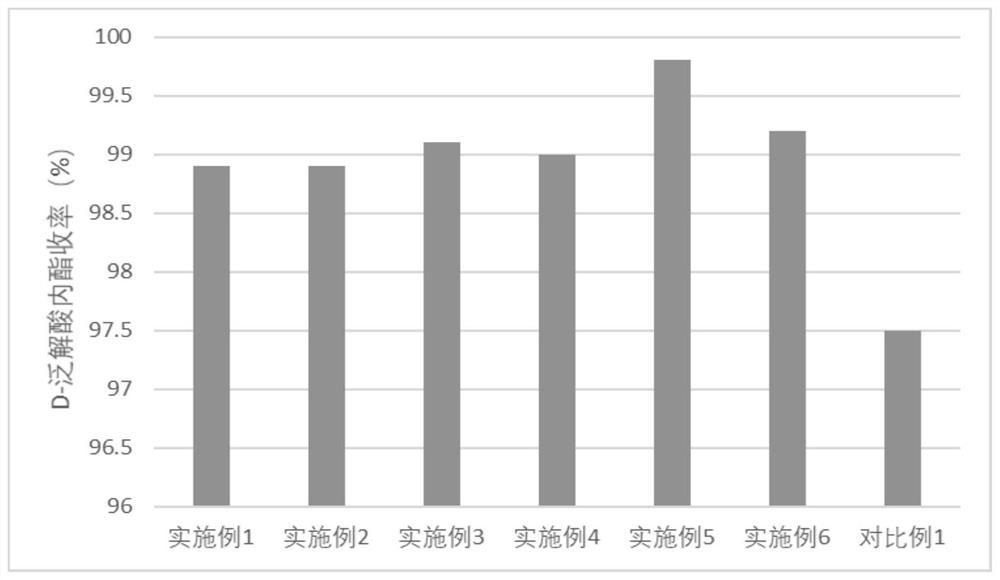
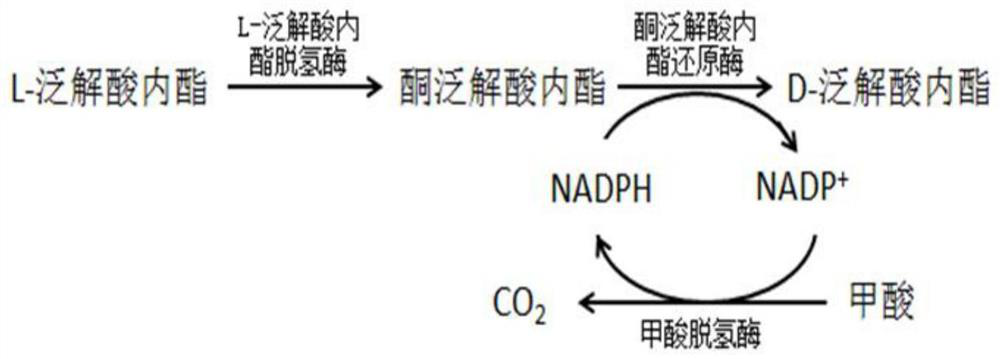
InactiveCN114457129AEfficient conversionImprove reaction efficiencyHydrolasesNucleic acid vectorKetoneFormate dehydrogenase H
The invention relates to a recombinant engineering bacterium and application thereof in efficient conversion of L-pantoic acid lactone, the recombinant engineering bacterium is induced to efficiently express L-pantoic acid lactone dehydrogenase, ketone pantoic acid lactone reductase and formate dehydrogenase, and the L-pantoic acid lactone is efficiently converted to generate DL-pantoic acid lactone. According to the recombinant engineering bacterium, the purity and quality of DL-pantoic acid lactone, the reaction efficiency and the resource utilization rate are remarkably improved, the production cost is reduced, and the recombinant engineering bacterium has the advantages of being easy and convenient to operate, environmentally friendly and the like.
Owner:ANHUI HUAHENG BIOTECH +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com