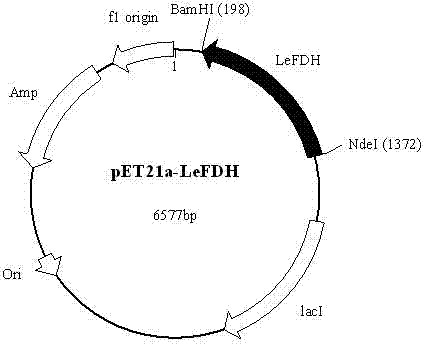
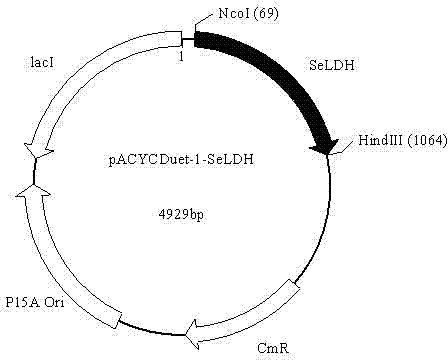
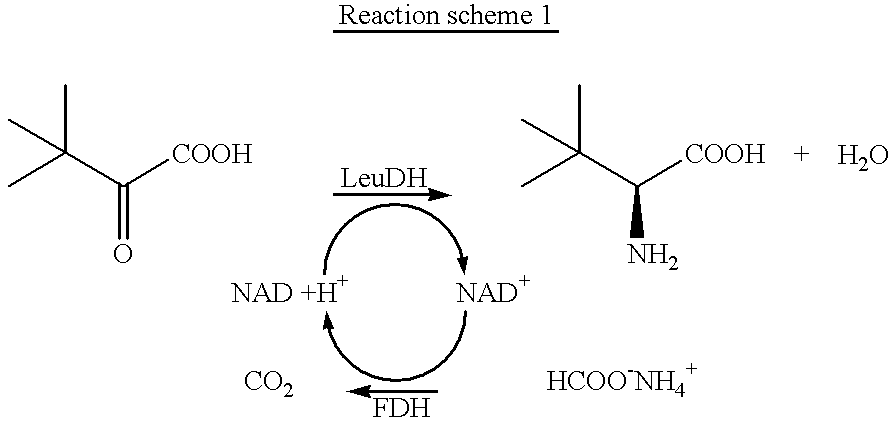
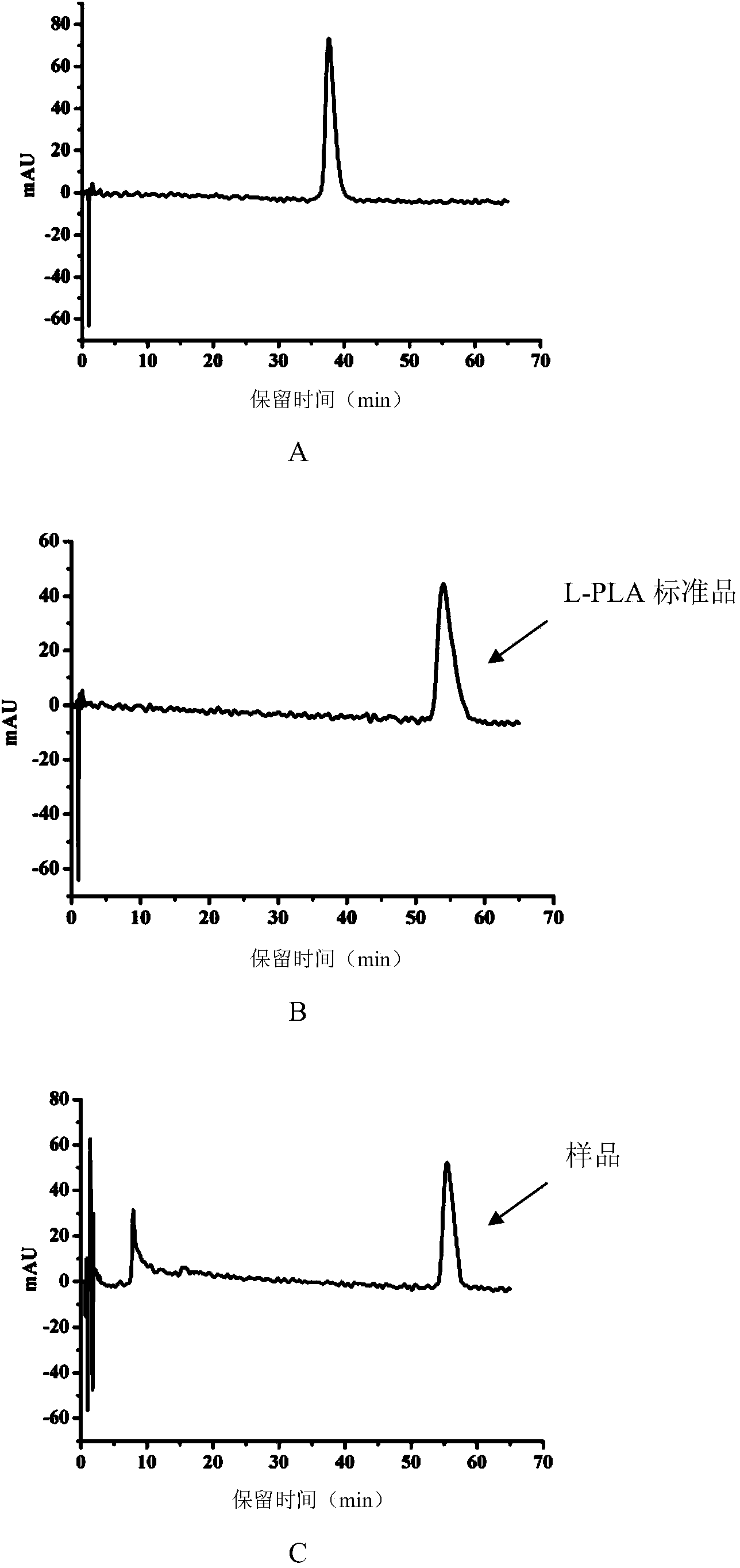
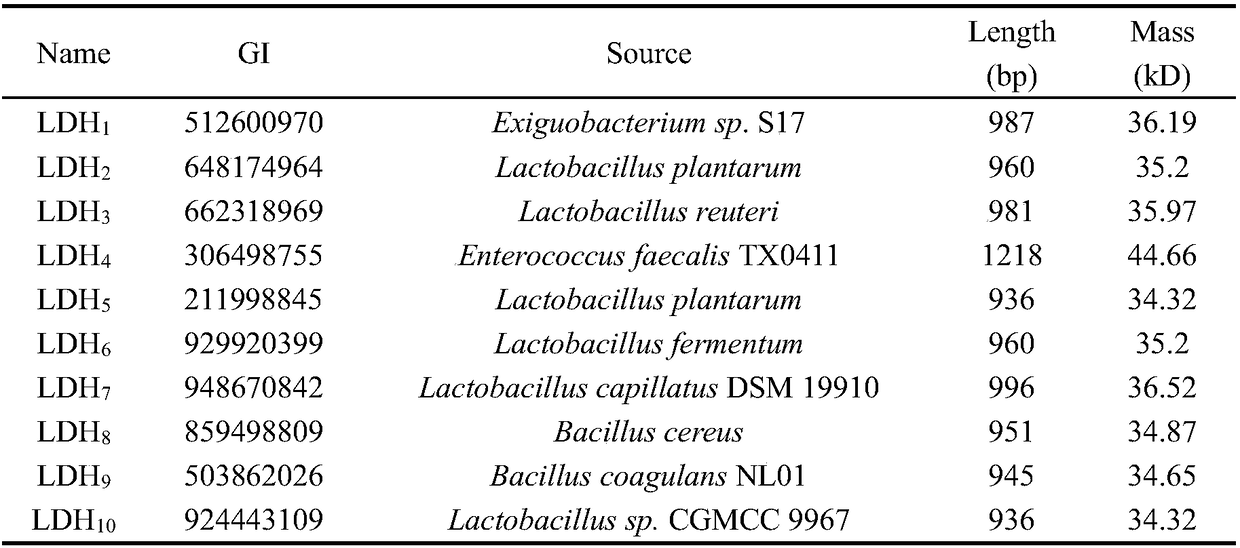
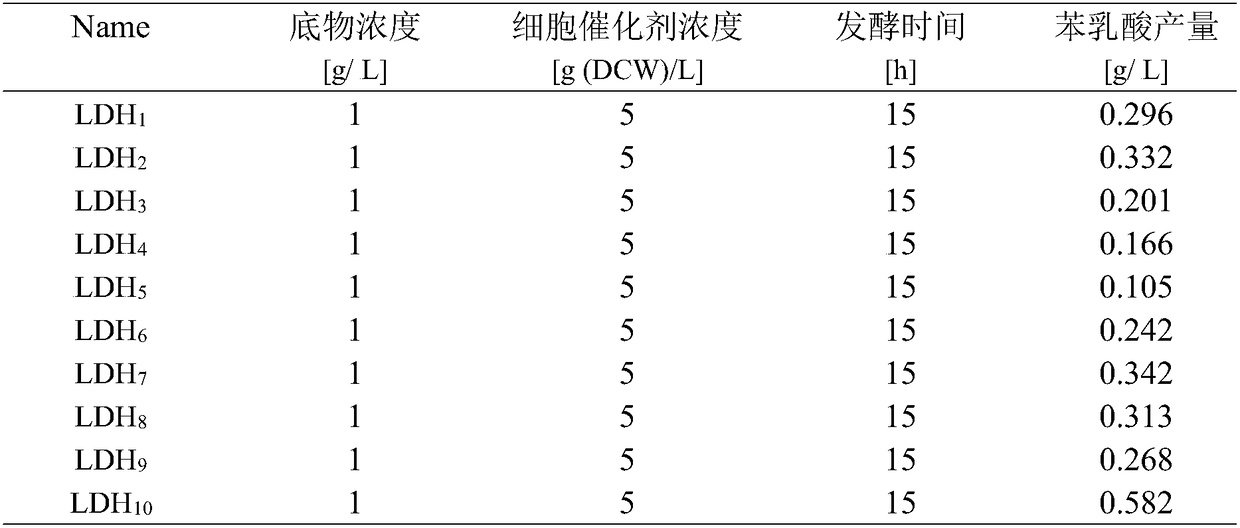
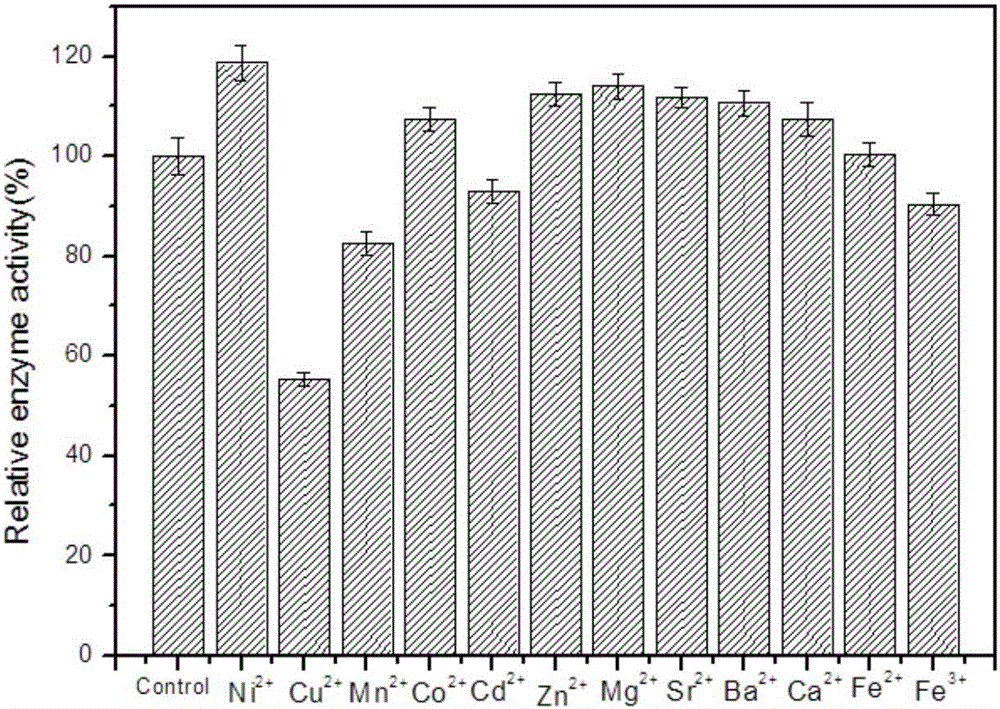
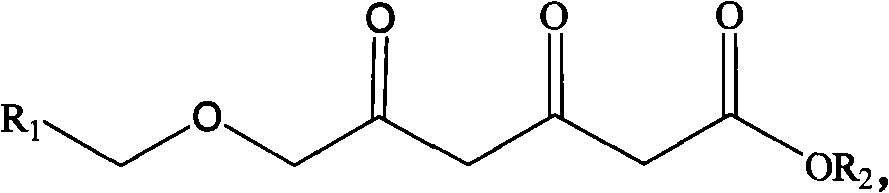
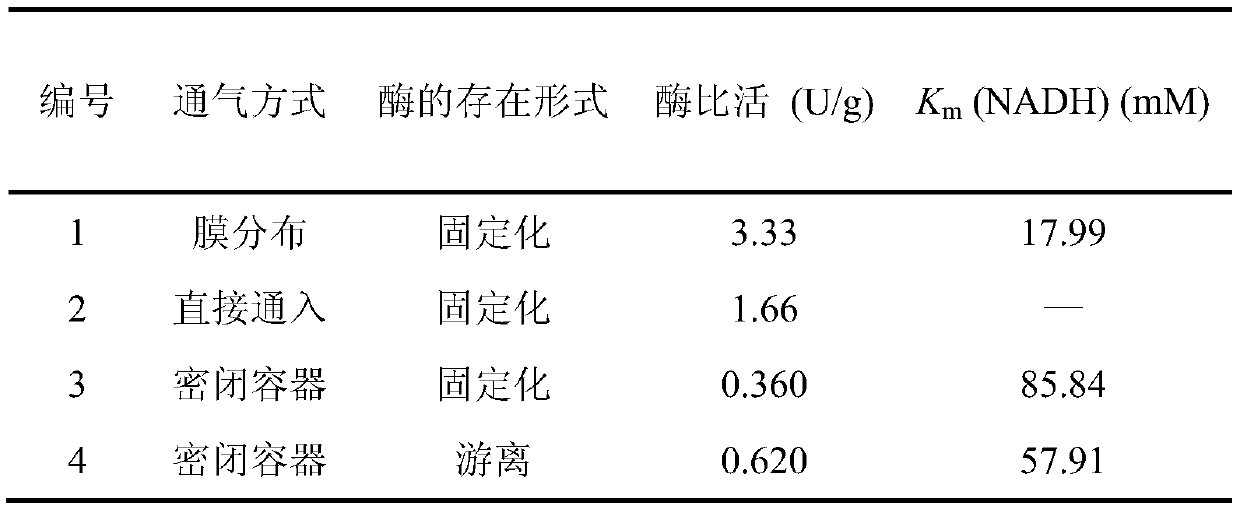
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
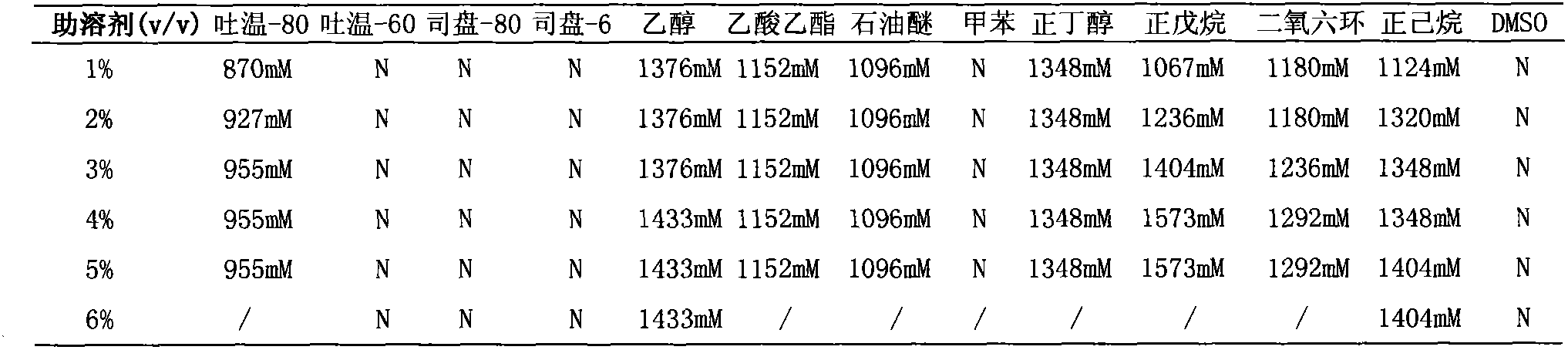
135 results about "Formate dehydrogenase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
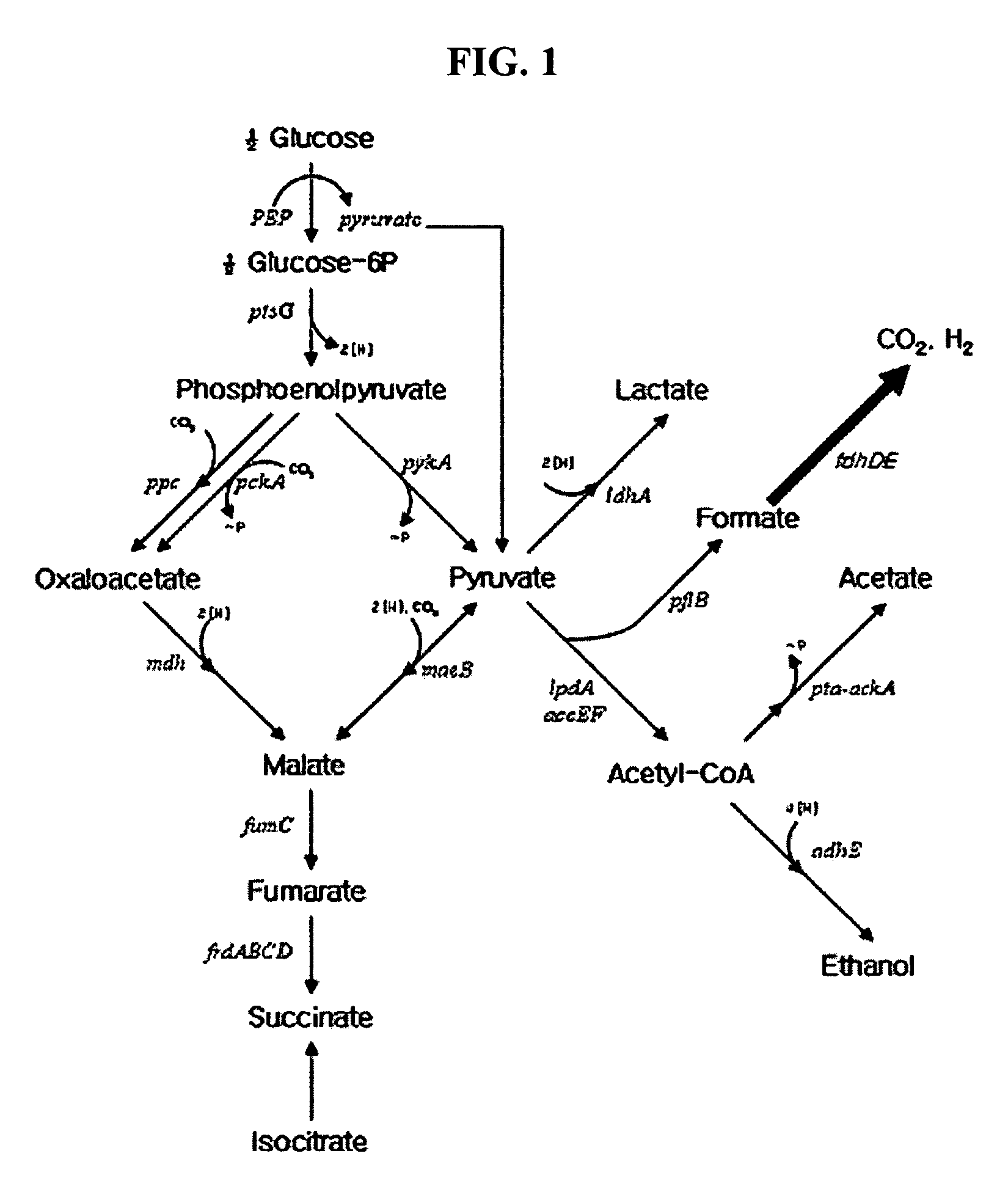
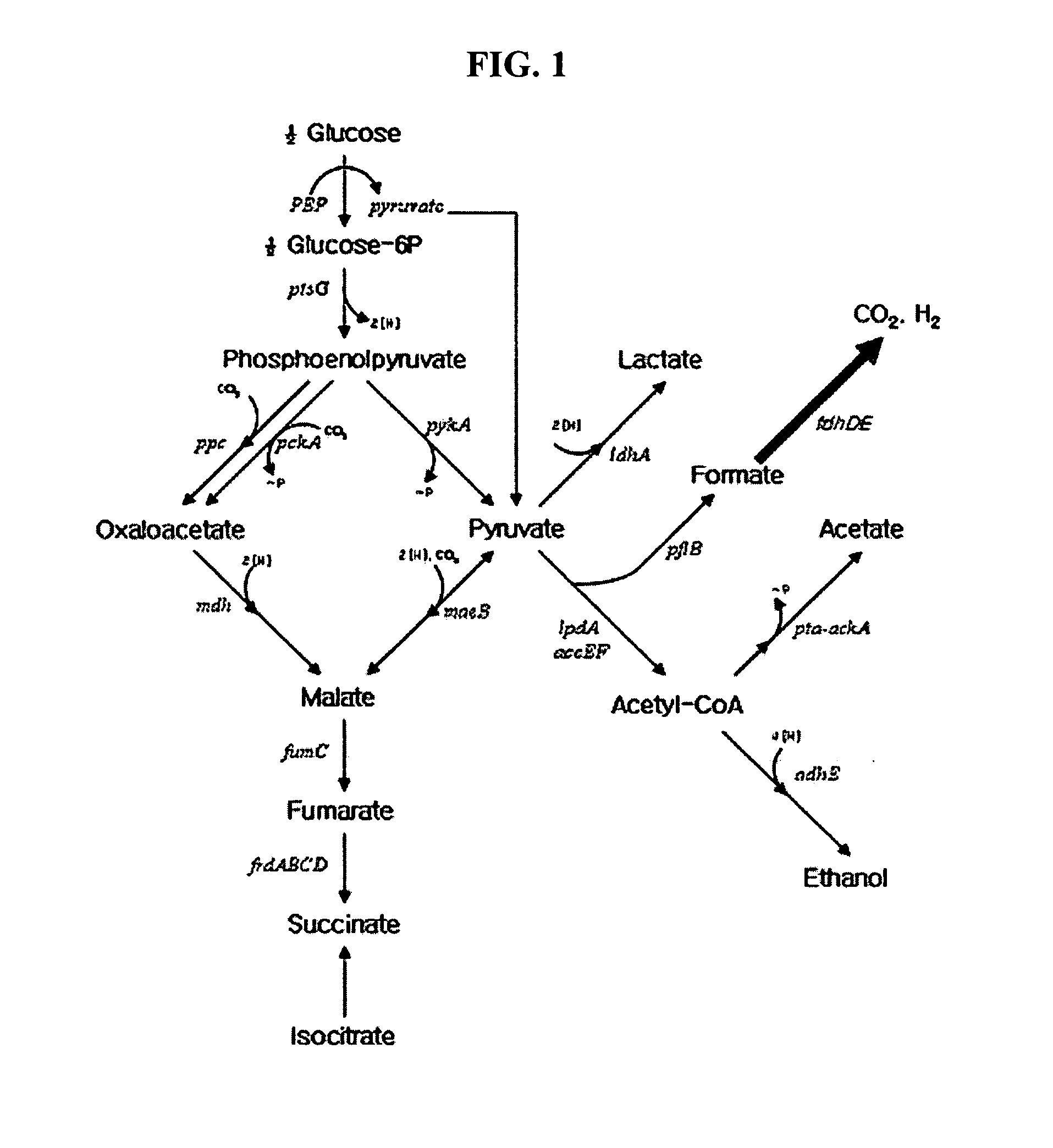
Formate dehydrogenases are a set of enzymes that catalyse the oxidation of formate to carbon dioxide, donating the electrons to a second substrate, such as NAD⁺ in formate:NAD+ oxidoreductase (EC 1.2.1.2) or to a cytochrome in formate:ferricytochrome-b1 oxidoreductase (EC 1.2.2.1).
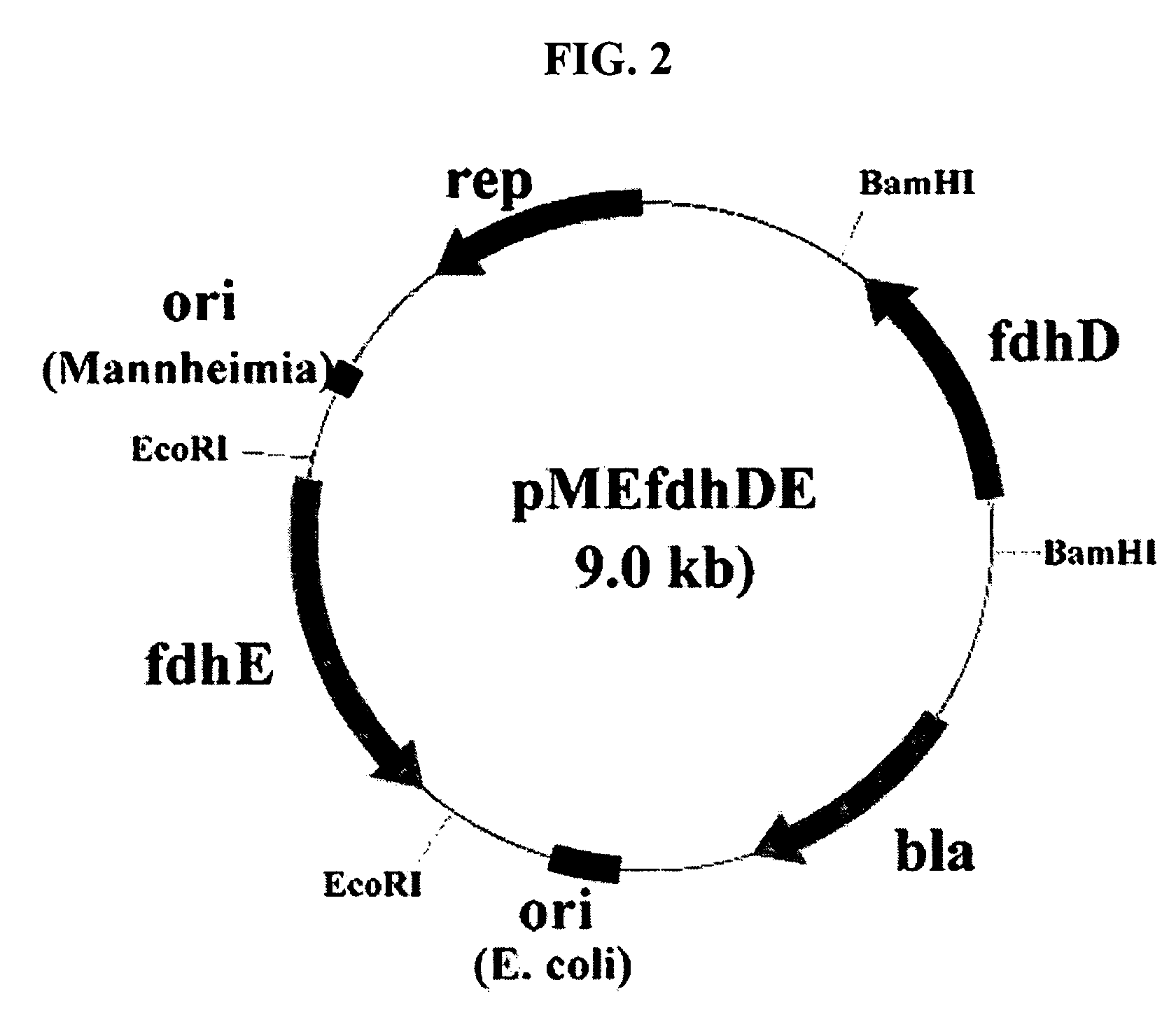
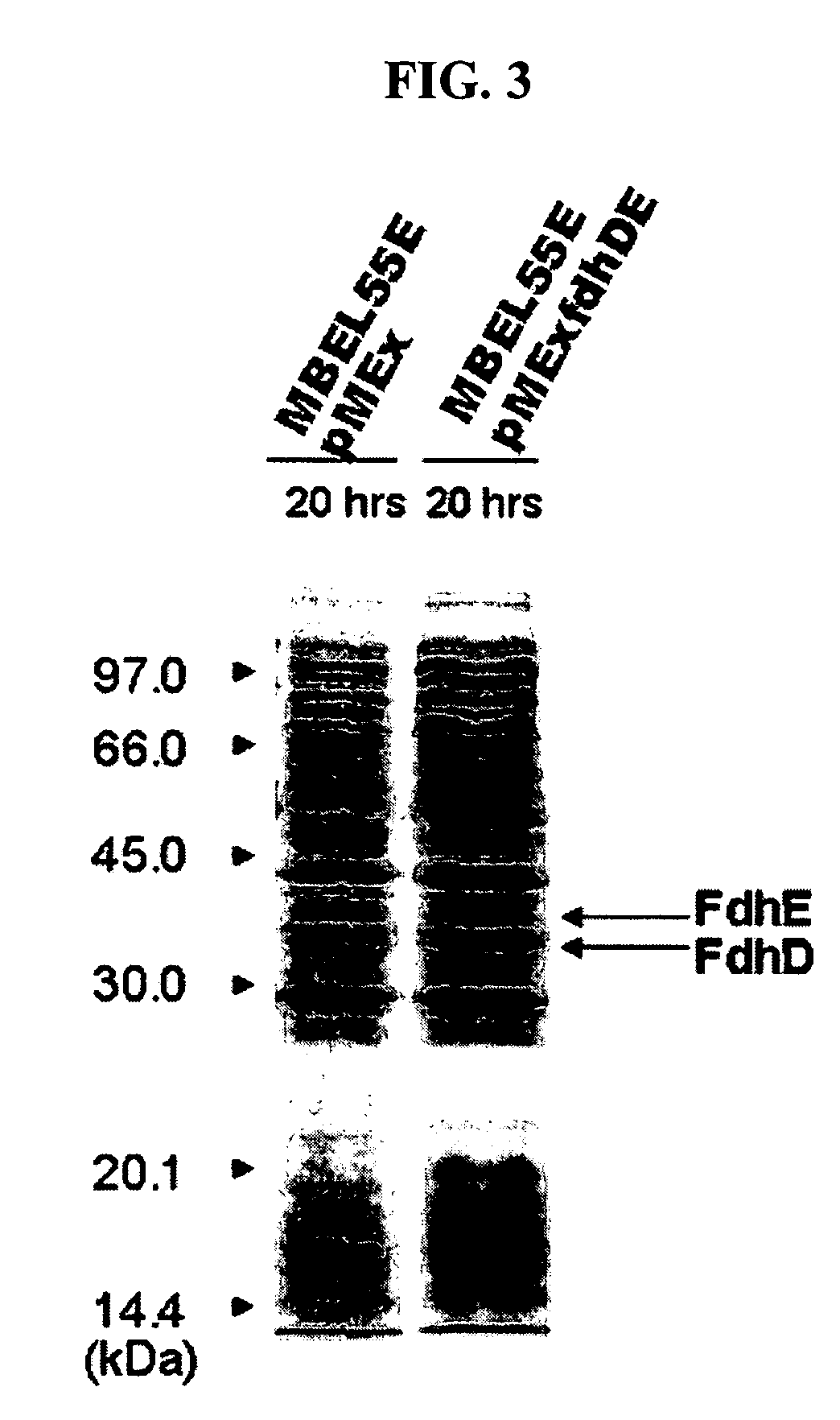
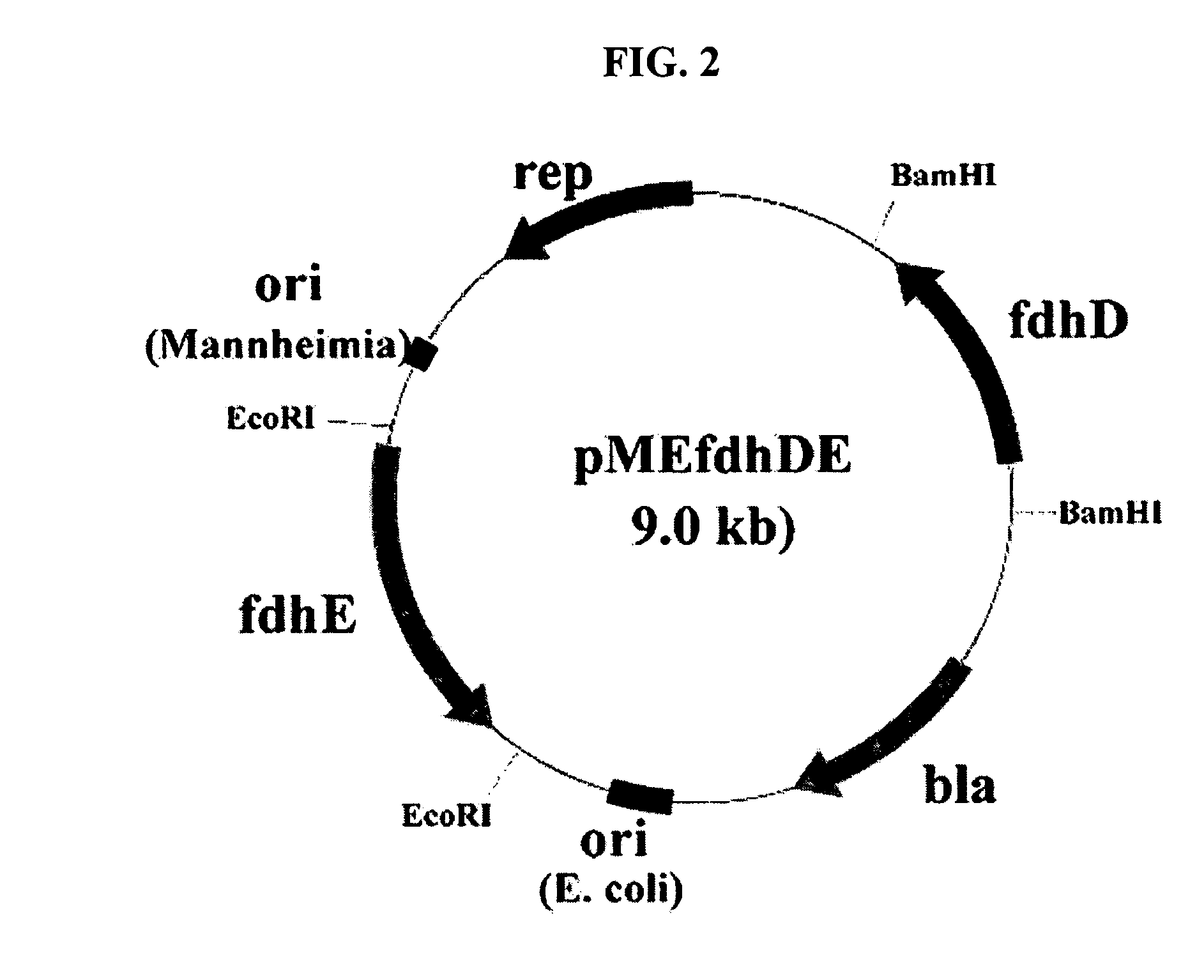
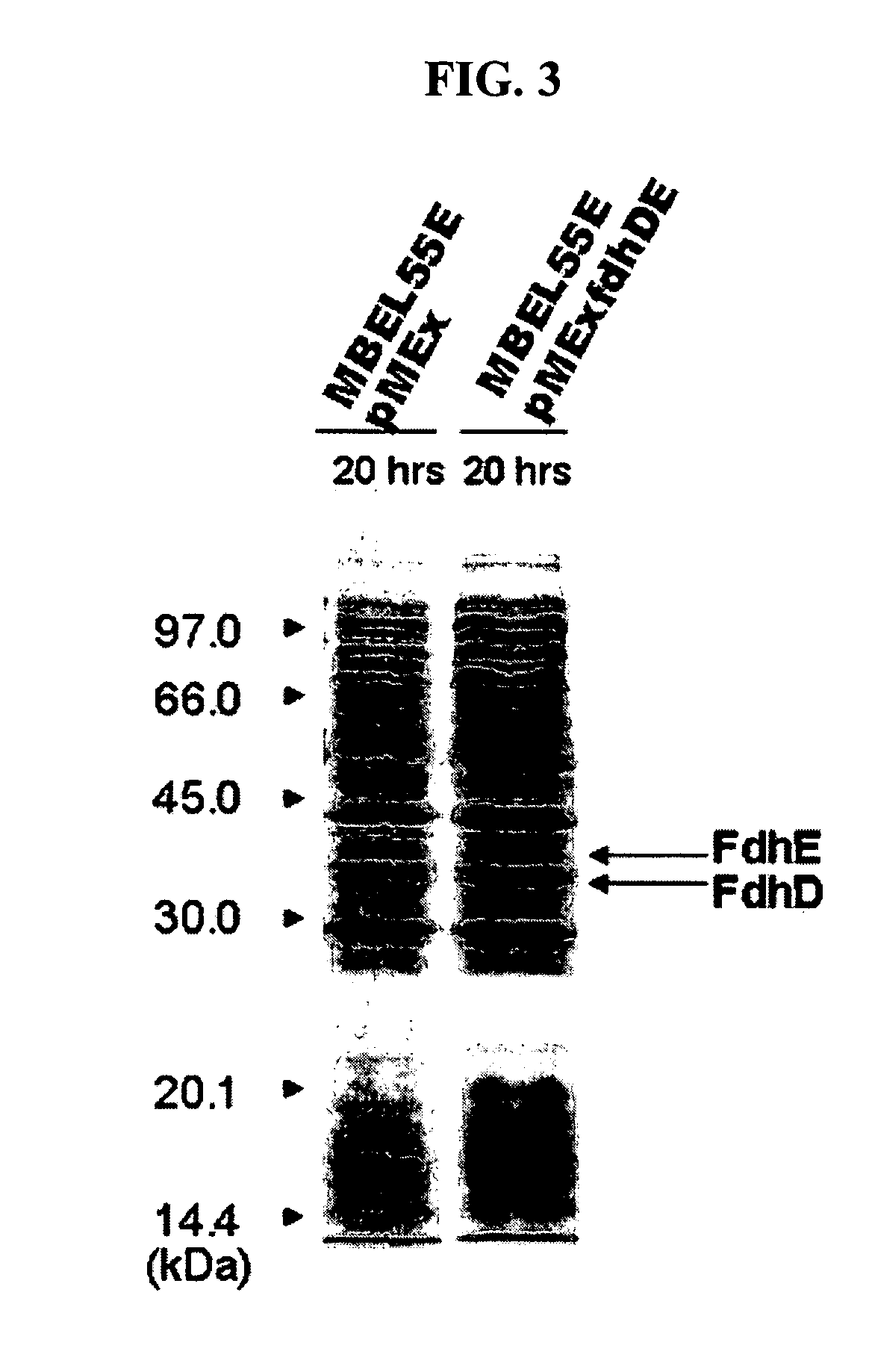
Gene encoding formate dehydrogenases D & E and method for preparing succinic acid using the same
Owner:KOREA ADVANCED INST OF SCI & TECH
Highly efficient hydrogen production method using microorganism
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH +1
Formate dehydrogenase mutant with improved enzyme activity and stability as well as construction method of formate dehydrogenase mutant
ActiveCN106479988APure enzyme specific enzyme activity increasedImprove toleranceBacteriaMicroorganism based processesHalf-lifeMutant
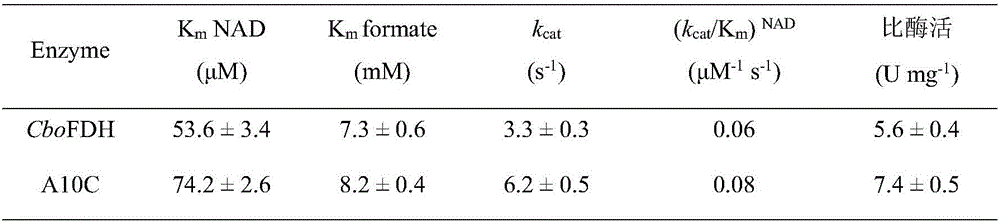
The invention discloses a formate dehydrogenase mutant with improved enzyme activity and stability as well as a construction method of the formate dehydrogenase mutant and belongs to the field of gene engineering technology. According to the mutant, on the basis of amino acid shown in SEQ ID No.2, alanine in the 10th site is mutated into cysteine. The enzyme ratio and the enzyme activity of the obtained mutant are increased by 1.3 times by comparison with those before mutation, the half-life period (t1 / 2) at the temperature of 60 DEG C is prolonged by 6.8 times by comparison with that before mutation, the tolerance of cupric ions is improved by 30 times than that before mutation, the stability under the condition that pH is equal to 4 is improved by 2.0 times, and the catalytic efficiency is improved by 1.4 times. The formate dehydrogenase mutant and the construction method indicate that an amino acid residue in the 10th site is mutated into cysteine, and then cysteine forms a correct disulfide bond with a 30th cysteine residue of natural formate dehydrogenase, so that the stability and the catalytic efficiency of the enzyme are improved, and the industrial application potential of the enzyme is improved.
Owner:JIANGNAN UNIV
Immobilized whole-cell catalyst and its preparation method and application
InactiveCN102260664ALow costEasy to operateBacteriaMicroorganism based processesPtru catalystLisinopril
The invention relates to the field of biotechnology in the pharmaceutical industry, and discloses the preparation of an immobilized whole-cell catalyst and its application in the synthesis of a Puley intermediate (R)-2-hydroxy-4-phenyl-butyric acid. The recombinant cells containing the D-type lactate dehydrogenase gene and the formate dehydrogenase gene are established and fixed to prepare the immobilized whole cell catalyst. Using this catalyst to synthesize the intermediate (R)-2-hydroxy-4-phenyl-butyric acid of lisinopril, the reaction conditions are mild, the action is specific, and there are no by-products. It is used in medicine and food industries. value.
Owner:SYNCOZYMES SHANGHAI
Mutants of formate dehydrogenase from Candida boidinii, new gene sequences encoding these and use of the new formate dehydrogenases
InactiveUS6242234B1Longer working lifetimeFungiMicroorganism based processesEscherichia coliWild type
The invention relates to new mutants of formate dehydrogenase from Candida boidinii, new gene sequences encoding these and use of the new formate dehydrogenases.The wild type FDH used hitherto in the industrial process for preparing amino acids becomes inactive after a certain time. New mutants of this wild type have been produced by targeted mutagenesis of a recombinant FDH from E. coli. The new mutants are preferably used in an enzymatic process for preparing chiral compounds.
Owner:EVONIK DEGUSSA GMBH
Alcohol dehydrogenase mutant and application thereof to synthesis of chiral biaryl alcohols
InactiveCN108359649AHigh stereoselectivityIncrease vitalityBacteriaMicroorganism based processesAlcoholMutant
The invention discloses an alcohol dehydrogenase mutant and an application thereof to synthesis of chiral biaryl alcohols, and belongs to the technical field of bioengineering. The alcohol dehydrogenase mutant has excellent catalytic activity and stereoselectivity and can be used for efficient catalysis for preparation of a series of R-configuration and S-configuration chiral biaryl alcohols. Alcohol dehydrogenase can be applied to synthesis of various chiral biaryl alcohol intermediates of antihistamine drugs by being coupled with glucose dehydrogenase or formate dehydrogenase. Compared withthe existing reports, a method for preparing the chiral biaryl alcohols from alcohol dehydrogenase through asymmetric catalytic reduction has the advantages of simple operation, high substrate concentration, complete reaction and high product purity, and has broad industrial application prospect.
Owner:JIANGNAN UNIV
Novel gene encoding formate dehydrogenases D & E and method for preparing succinic acid using the same
Nucleotide sequences encoding formate dehydrogenases D & E and a method for preparing succinic acid using the same, more particularly, formate dehydrogenases D & E converting formate to carbon dioxide and hydrogen, fdhD and fdhE nucleotide sequences encoding the formate dehydrogenases D & E, recombinant vectors containing the nucleotide sequences, microorganisms transformed with the recombinant vectors, and a method for preparing succinic acid using the transformed microorganism.
Owner:KOREA ADVANCED INST OF SCI & TECH
Highly efficient hydrogen production method using microorganism
ActiveUS20060128001A1Highly efficient biological hydrogen generation capabilityIncrease the number ofFungiBacteriaMicroorganismCompound (substance)
It is intended to obtain anaerobic microbial cells in an amount sufficient for hydrogen generation reaction, impart a hydrogen generation function to an aerobic microorganism within a short time and provide an industrial advantageous method of producing hydrogen. The above object can be established by providing a highly efficient microbial hydrogen production method characterized by comprising culturing a microorganism having a formate dehydrogenase gene and a hydrogenase gene under aerobic conditions, culturing the resulting microbial cells under anaerobic conditions in a liquid culture medium containing a formic acid compound, and then using the thus obtained cells for hydrogen generation.
Owner:RES INST OF INNOVATIVE TECH FOR THE EARTH +1
Kit for diagnosing diseases in system of liver and gall
InactiveCN101003831AExtended storage timeUndisturbedMicrobiological testing/measurementBiological testingDisease causePolymer
This invention relates to a test kit for diagnosing hepatic and biliary diseases. The test kit has such advantages as high stability, high accuracy and high sensitivity. The test kit comprises two reagents. Reagent 1 comprises oxidized thio coenzyme, buffer solution, preservative, and anti-interference agent. Reagent 2 comprises 3alpha-HSD, reduced coenzyme, buffer solution, preservative, complexing agent, polymer accelerator, stabilizer, and one of formate dehydrogenase-formic acid-NAD, glutamate dehydrogenase-glutamic acid-oxidized nicotinamide coenzyme, glucose-6-phosphate dehydrogenase-glucose-oxidized nicotinamide coenzyme, glucose dehydrogenase-xylose-oxidized nicotinamide coenzyme, lactate dehydrogenase-alpha-ketoglutarate-oxidized nicotinamide coenzyme, and glycerol dehydrogenase-ethylene glycol-oxidized nicotinamide coenzyme.
Owner:王贤理
Process for preparing dipeptidyl peptidase IV inhibitors and intermediates therefor
InactiveUS20050260712A1Procedure of process can be improvedReduce processing timeSugar derivativesBacteriaPhenylalanine dehydrogenaseDipeptidyl peptidase
A process for production of cyclopropyl-fused pyrrolidine-based inhibitors of dipeptidyl peptidase IV is provided which employs a BOC-protected amine of the structure prepared by subjecting an acid of the structure to reduce amination by treating the acid with ammonium formate, nicotinamide adenine dinucleotide, dithiothreitol and partially purified phenylalanine dehydrogenase / formate dehydrogenase enzyme concentrate (PDH / FDH) and without isolating treating the resulting amine of the structure 2 with di-tert-butyl dicarbonate to form the BOC-protected amine.
Owner:ASTRAZENECA AB
Construction of coenzyme efficient regeneration system and application thereof
InactiveCN105238807AImprove conversion efficiencyLow costFermentationVector-based foreign material introductionEscherichia coliThreonine
The invention relates to a method for preparing alpha-aminobutyric acid by utilizing series connection amino acid dehydrogenase and codon optimization formate dehydrogenase recombinant escherichia coli. First, codon optimization is performed on a Candida boidinii formate dehydrogenase gene sequence according to the codon preference of the escherichia coli. After codon optimization on formate dehydrogenase, the expression quantity of formate dehydrogenase in the escherichia coli can be improved remarkably, the yield of alpha-aminobutyric acid is increased, and in the escherichia coli, co-expression formate dehydrogenase and L-amino acid dehydrogenase can promote circulation of cofactors in mycetome without needing the adding of any exogenous cofactors. In addition, the process of utilizing whole-cell transformation bulk chemical L-threonine to produce alpha-aminobutyric acid is simple and quick, and the cost is low. In a 5 L fermentation tank, the yield of alpha-aminobutyric acid obtained through the method can be 81.5 g / L, and an actually effective strategy is provided for industrial production of alpha-aminobutyric acid.
Owner:ANHUI HUAHENG BIOTECH
Escherichia coli with coexpression of L-lactate dehydrogenase and formate dehydrogenase as well as construction method and application of escherichia coli
ActiveCN104130967ANotable featuresRemarkable effectBacteriaMicroorganism based processesEscherichia coliL-Lactate dehydrogenase
The invention discloses escherichia coli with coexpression of L-lactate dehydrogenase and formate dehydrogenase. The escherichia coli is characterized in that L-lactate dehydrogenase genes ldhL from bacillus coagulans and formate dehydrogenase genes fdh from candida boidinii are introduced into the escherichia coli, wherein the register number of GenBank of nucleotide sequences of the L-lactate dehydrogenase genes ldhL is KF386111; the register number of GenBank of nucleotide sequences of the formate dehydrogenase genes fdh is AJ011046. The invention further discloses a construction method and an application of the reconstructed escherichia coli. The method has the characteristics of being simple to operate, low in cost, high in product synthesis efficiency and high in optical purity. Thus, an effective way for biosynthesis of L-phenyllactic acid is provided.
Owner:深圳市华利康纤生物科技有限公司
Method for producing phenyllactic acid through whole-cell transformation of phenylalanine
InactiveCN108277190AThe solution steps are cumbersomeLow resolutionBacteriaMicroorganism based processesChemical synthesisPhenylalanine
The invention discloses a method for producing a phenyllactic acid through whole-cell transformation of phenylalanine, and belongs to the technical field of enzyme engineering. The method successfullyconstructs an L-amino acid deaminase, L-lactic dehydrogenase and formate dehydrogenase co-expressed strain. Phenyllactic acid of 30 g / L is produced through the whole-cell transformation of phenylalanine, and the transformation rate is 100%. The establishment of a whole-cell transformation system solves the problems of complicated steps, low yield and environment pollution in the chemical synthesis of the phenyllactic acid as well as the problem of low transformation rate in the production of the alpha-oxoglutarate through the enzymatic conversion, the phenyllactic acid is produced through a one-step method at high yield without pollution, and a certain theoretical foundation is laid for the subsequent industrial production.
Owner:JIANGNAN UNIV
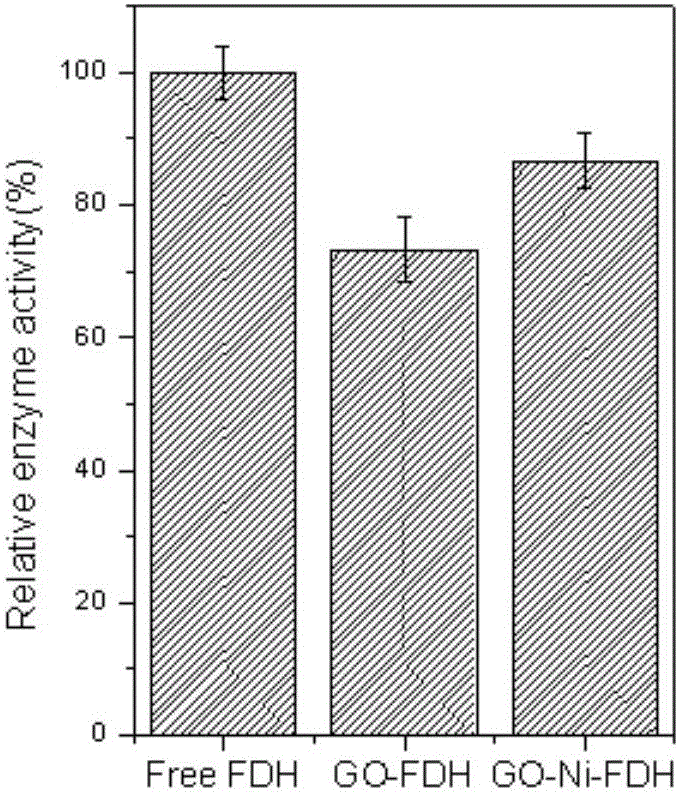
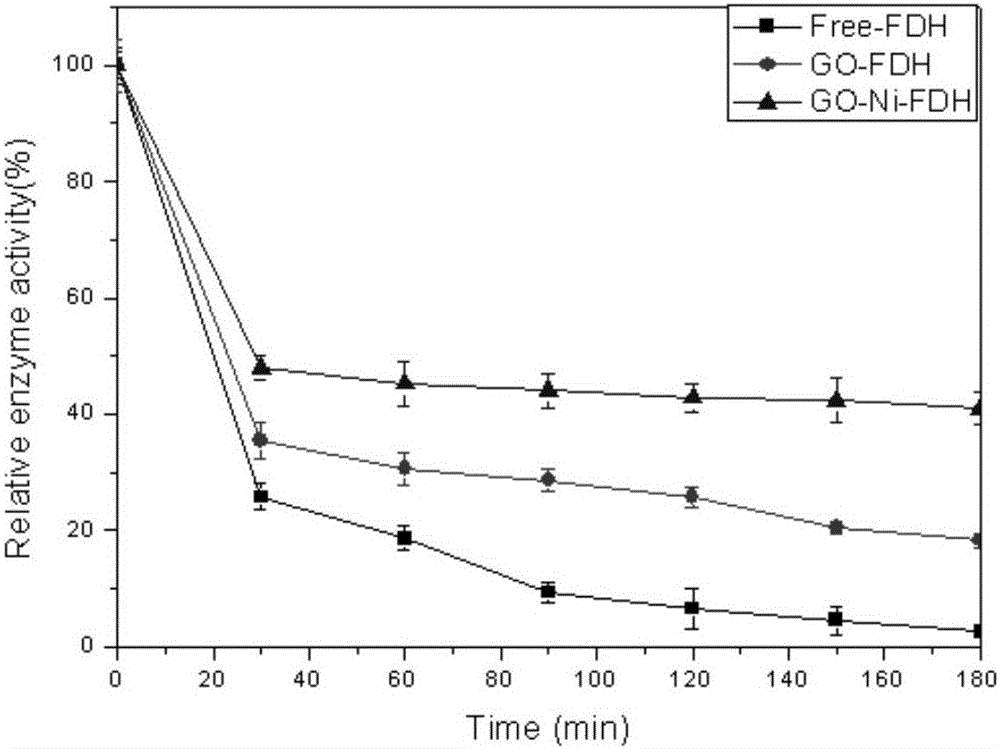
Method of immobilizing enzyme by means of coordination of graphene oxide and metal ions
ActiveCN106191025AEvenly dispersedStable directional immobilizationOn/in inorganic carrierFormate dehydrogenaseHistidine
The invention discloses a method of immobilizing enzyme by means of coordination of graphene oxide and metal ions and belongs to the technical field of enzyme immobilization. In the immobilization, transition-state metal ions are coordinated with a carboxyl group on the graphene oxide, and through the coordination, formate dehydrogenase is immobilized. The graphene oxide in a lamellar structure is an excellent immobilization carrier, of which the surface has functional groups, such as epoxy groups, carbonyl groups, carboxyl groups and the like, so that the carrier is large in support area. Through the coordination effect of the metal ions, the graphene oxide is uniformly dispersed and support load of the enzyme is increased. In addition, through a coordination interaction with a histidine label of the enzyme, the enzyme is immobilized on the graphene oxide. The method is simple in processes, has mild preparation conditions and high immobilization rate. The immobilized enzyme is significantly improved in temperature stability, pH stability and repeatedly usage stability, and is high in enzyme activity recovery rate.
Owner:XIAMEN UNIV
Thermostability enhanced formate dehydrogenase mutant and preparation method thereof
ActiveCN104342406AImprove thermal stabilityNo significant decrease in activityBacteriaMicroorganism based processesWild typeFormate dehydrogenase
The invention relates to a thermostability enhanced formate dehydrogenase mutant and a preparation method thereof. The mutant is derived from wild type formate dehydrogenase of Candida boidinii, can catalyze coenzyme NADH cyclic regeneration, shows higher thermostability compared with the wild type formate dehydrogenase, and has one or more mutation characteristics of I98V, V152I, A154D and D158R. The recombinant formate dehydrogenase mutant provided by the invention has better thermostability, and is suitable for cyclic regeneration of NADH under high temperature. Compared with the formate dehydrogenase discovered so far, the formate dehydrogenase mutant has the activity that is not significantly reduced in the range of 50DEG C-55DEG C, and has better thermostability, thus being more suitable for industrial application.
Owner:常州百奥生物科技有限公司
Method for producing L-2-aminobutyric acid by double immobilized multi-enzyme systems
InactiveCN102517351AImprove recycling ratesImprove regeneration efficiencyChemical industryFermentationEnzyme systemOxidative enzyme
The invention discloses a method for producing L-2-aminobutyric acid by double immobilized multi-enzyme systems. The method provided by the invention comprises the following steps that 1, threonine dehydrogenase and leucine dehydrogenase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized multi-enzyme system is obtained; and 2, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase are fixed on reversely-dissoluble pH-sensitive polymer carriers so that a co-immobilized coenzyme regeneration system is obtained. The method for producing L-2-aminobutyric acid by the double immobilized multi-enzyme systems utilizes dissolution reversibility of co-immobilized enzymes, realizes effective separation of the co-immobilized enzymes and products, improves accessibility between the co-immobilized enzymes and reactants, improves recovery and utilization rates of threonine dehydrogenase, leucine dehydrogenase, alcohol oxidase, formaldehyde dehydrogenase and formate dehydrogenlyase, improves coenzyme regeneration efficiency, reduces follow-up separation purification processes, simplifies a process flow and reduces a production cost.
Owner:李鑫
Preparation method of (R)-2-(2,5-difluorophenyl) pyrrolidine or salt thereof
ActiveCN109593802ASignificant technological progressImprove stabilityFermentationGrignard reagentGrignard reaction
The invention provides a preparation method of (R)-2-(2,5-difluorophenyl) pyrrolidine or salt thereof. N-protected pyrrolidone and a 2,5-fluorophenyl magnesium chloride Grignard reagent are subjectedto a Grignard reaction, and a compound represented as the formula 3 is obtained; the compound represented as the formula 3 is subjected to deprotection and ring closure under the acidic condition, anda compound represented as the formula 4 or salt of the compound is obtained; under enzyme catalysis, formic acid or formate is utilized as a hydrogen donor, a cofactor is adopted, cofactor cycle is realized, and the compound represented as the formula 4 or the salt of the compound is selectively reduced to synthesize an optically pure compound, namely, (R)-2-(2,5-difluorophenyl) pyrrolidine or the salt thereof; enzyme is a combination of recombinant imine reductase and formate dehydrogenase, and the cofactor is oxidized / reduced type nicotinamide adenine dinucleotide phosphate or oxidized / reduced type nicotinamide adenine dinucleotide. The method is safe in process and simple to operate, and yield and optical purity of a product are both higher.
Owner:SHANGHAI UNIV OF MEDICINE & HEALTH SCI
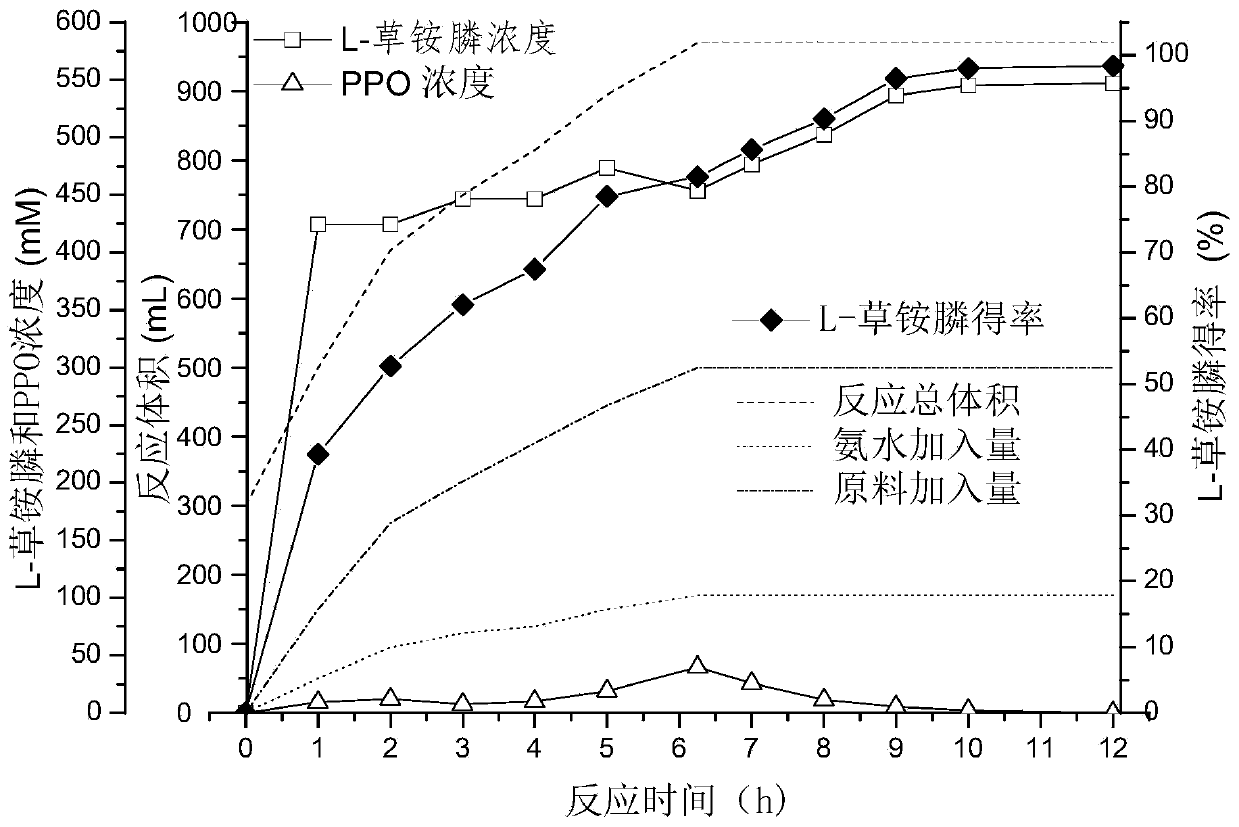
Enzyme combination for producing L-phosphinothricin, and production method of L-phosphinothricin
InactiveCN111139270AReduce manufacturing costTake full advantage of catalytic activityOxidoreductasesFermentationPhosphorous acidPhosphite dehydrogenase
The invention discloses an enzyme combination for producing L-phosphinothricin. The enzyme combination comprises glutamate dehydrogenase and a coenzyme regenerating enzyme, wherein the coenzyme regenerating enzyme is alcohol dehydrogenase, formate dehydrogenase and phosphite dehydrogenase. The invention also discloses a production method of L-phosphinothricin, 4-(methylhydroxyphosphinyl)-2-oxobutyric acid is used as a raw material, NH<4><+>, a coenzyme NADP<+> / NADP, and a coenzyme regeneration substrate are added, and then the enzyme combination is used for catalysis, wherein the glutamate dehydrogenase is used to catalyze a reaction of 4-(methylhydroxyphosphinyl)-2-oxobutyric acid to obtain L-phosphinothricin, and the coenzyme regenerating enzyme is used to reduce NADP<+> to NADP. According to the enzyme combination and the production method of L-phosphinothricin provided by the invention, by-products produced are very easy to remove, a post-treatment process of the product is simplified, the total yield of the product is greater than 95%, and the production cost of L-phosphinothricin is reduced, so that the method is a green, environment-friendly, and low-carbon process route, and is suitable for large-scale industrial production applications.
Owner:ZHEJIANG UNIV
Construction and application of Escherichia coli engineering bacterium for immobilizing CO2 and producing malic acid
ActiveCN110951660APromote accumulationIncrease productionBacteriaTransferasesEscherichia coliPhosphorous acid
The invention discloses construction and application of Escherichia coli engineering bacterium for immobilizing CO2 and producing malic acid, and belongs to the field of fermentation. The engineeringbacterium is a strain obtained by carrying out gene engineering modification on Escherichia coli MG1655. The genetic engineering transformation is as follows: 1, knocking out a fumarate reductase gene, a fumarase gene, a lactic dehydrogenase gene and an ethanol dehydrogenase gene, and carrying out free over-expression on formate dehydrogenase, acetyl coenzyme A synthetase, acylated acetaldehyde dehydrogenase, formaldehyde lyase, dihydroxyacetone kinase, malic enzyme and phosphorous acid oxidordeuctase so as to obtain a strain GH0407. The strain is used for fermentation production of malic acid, CO2 and glucose are used as co-substrates for anaerobic fermentation for 72 h, the malic acid yield reaches 39 g / L, the yield is 1.53 mol / mol, and malic acid is not accumulated in an original starting strain.
Owner:JIANGNAN UNIV
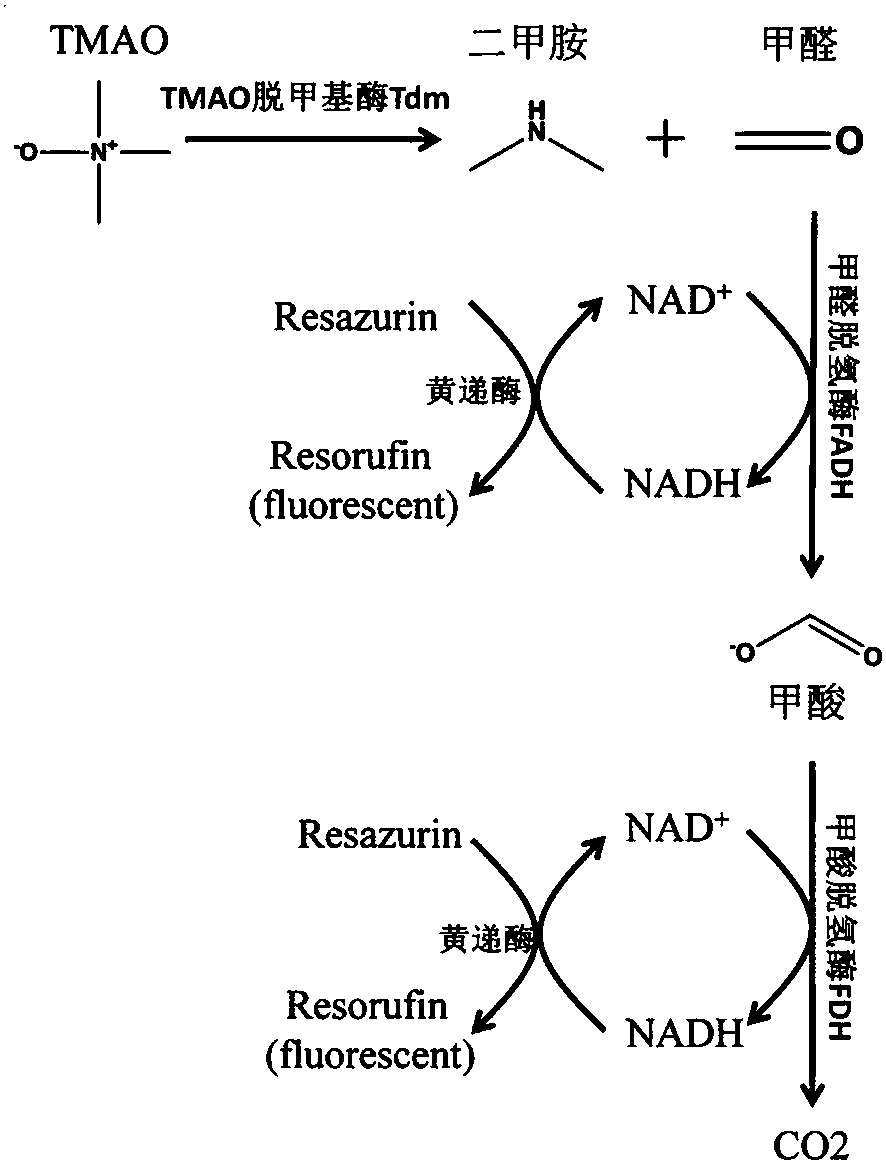
Method for detecting TMAO (trimethylamine oxide) by enzyme method and application thereof
ActiveCN108507984AHigh sensitivityRapid and stable detectionFluorescence/phosphorescenceNad dependent dehydrogenaseFluorescence
The invention discloses a method for detecting TMAO (trimethylamine oxide) by an enzyme method and application thereof. The detection method utilizes a multi-enzyme combination system containing TMAOTDM (demethylase), FADH (formaldehyde dehydrogenase), FDH (formate dehydrogenase) and diaphorase, so as to perform fluorescence determining on the TMAO; the method can be used for developing a TMAO detection kit. The method for detecting the TMAO by the enzyme method has the advantages that the defect of failure to directly determine based on dehydrogenase and diaphorase coupling because of no participation of NAD dependence dehydrogenase in the metabolism path of the TMAO is overcome; by adopting the multi-enzyme coupling of TDM-FADH-FDH-diaphorase, the system for fluorescence determining ofthe TMAO is constructed, and one TMAO molecule is decomposed into two fluorescence signals; the sensitivity is high, the detection is rapid and stable, the repeatability is good, the operation is simple and convenient, and the cost is lower.
Owner:THE SECOND HOSPITAL OF SHANDONG UNIV
Biotransformation method for L-glufosinate
InactiveCN107630052ASimple processSuitable for industrial productionFermentationSolventGenetically engineered
The invention discloses a biotransformation method for L-glufosinate. The biotransformation method comprises the following steps: composing a reaction system from a solvent and 2-carbonyl-4-(hydroxymethylphosphoryl)butyric acid used as a substrate, and then adding a biocatalyst, coenzyme, an additive and an ammonium salt into the reaction system for a biotransformation reaction so as to obtain a transformed solution containing L-glufosinate, wherein the biocatalyst is the whole cell of a genetically engineered bacterium co-expressed by glufosinate dehydrogenase derived from Saccharomyces cerevisia and formate dehydrogenase derived from Candida boidinii; the coenzyme is NADP+; and the ammonium salt is ammonium formate. The biotransformation method is simple in process flow, free of specialrequirements on equipment and suitable for industrial production. HPLC-MS and HPLC are employed for monitoring until the substrate is fully utilized.
Owner:武汉茵茂特生物技术有限公司
Enhancement of Microbial Ethanol Production
InactiveUS20090226992A1Easy to transformEfficient fermentationSugar derivativesBacteriaLactate dehydrogenaseDNA construct
A thermophilic microorganism lacks lactate dehydrogenase activity and preferably contains an active pyruvate formate lyase pathway. The thermophilic microorganism contains a gene encoding an NAD-linked formate dehydrogenase. The gene encoding an NAD-linked formate dehydrogenase is preferably a codon optimised version of the gene encoding a thermostable NAD-linked formate dehydrogenase. DNA constructs allow stable expression of the gene encoding an NAD-linked formate dehydrogenase in the thermophilic microorganism. The DNA constructs are based upon use of an insertion sequence to achieve stable expression or recombination to insert the gene encoding an NAD-linked formate dehydrogenase into the lactate dehydrogenase gene, thus achieving gene knockout and new functionality in a single step. The microorganisms are useful in fermentation of sugars to produce ethanol.
Owner:BIOCONVERSION TECH LTD
Single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of single cell factory
ActiveCN108103038AOptimize regeneration rateReduce the cost of trainingMicroorganism based processesOxidoreductasesEscherichia coliCell factory
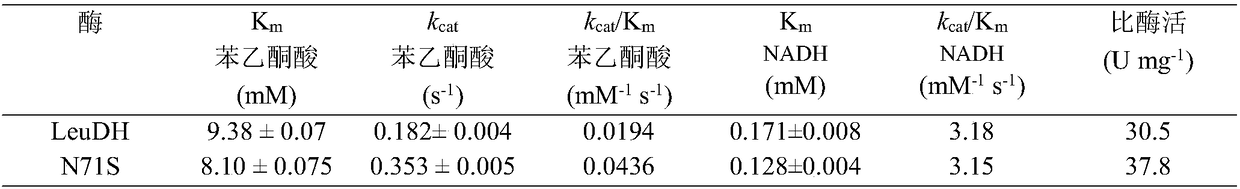
The invention discloses a single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of the single cell factory and belongs to the technical fieldof microorganisms. Firstly, efficient expression of leucine dehydrogenase obtained from Bacillus cereus in escherichia coli is realized, and site-directed mutation is carried out to obtain a mutant N71S with a remarkably improved reduction property; a mutant enzyme and a formate dehydrogenase mutant are co-expressed in the escherichia coli to form an intracellular in-situ co-factor NADH (Nicotinamide Adenine Dinucleotide) circulating system; the expression amount of the formate dehydrogenase mutant is optimized and controlled through a promoter and an RBS (Ribosomal Binding Site) sequence to successfully construct a recombinant escherichia coli single cell factory; the single cell factory is subjected to whole-cell conversion to prepare the L-phenylglycine. The method disclosed by the invention has the advantages of simple and rapid conversion process, low cost, no byproduct and easiness for separation and purification; when conversion is carried out in a 5L fermentation tank for 4h, the yield of the L-phenylglycine can reach 105.7g / l, the conversion rate is 93.3 percent and the space-time yield of the L-phenylglycine is 26.3g / L; an actually practical and effective strategy is provided for industrial production of the L-phenylglycine.
Owner:JIANGNAN UNIV
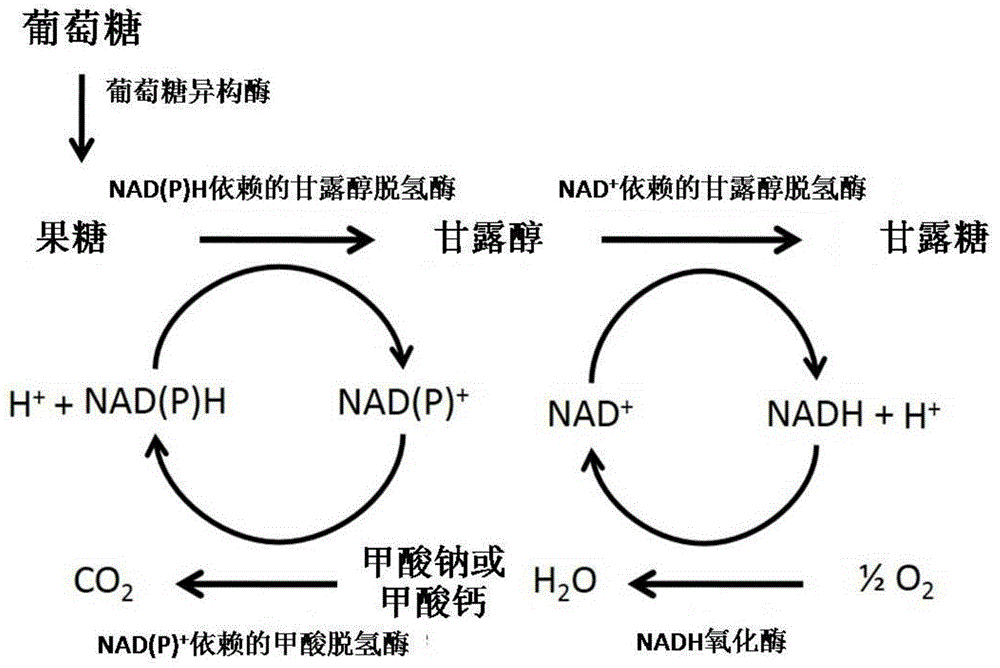
Method for preparing mannose through biological process
The invention discloses a method for preparing mannose through a biological process. According to the method, one or two of sodium formate and calcium formate, and one or two of glucose and fructose are taken as the raw materials, under the condition that glucose isomerase is available or not available, a biological catalysis system composed of an NAD (P)H-dependent mannitol dehydrogenase, an NAD(P)<+>-dependent formate dehydrogenase, an NAD<+>-dependent mannitol dehydrogenase, an NADH oxidase and a coenzyme is adopted for catalyzing, so that the mannose is prepared. The method for preparing the mannose provided by the invention has the characteristics that the raw materials are cheap, the reaction selectivity is high, etc, and the method belongs to a novel preparation reaction route of the mannose.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing chiral (S)-acetoin by virtue of whole-cell biological catalysis
InactiveCN105969812ALow costImprove regenerative abilityBacteriaMicroorganism based processesEscherichia coliDrug biotransformation
The invention discloses a method for preparing chiral (S)-acetoin by virtue of whole-cell biological catalysis. The method comprises the following steps: firstly carrying out coexpression on butanedione reductase genes and formate dehydrogenase genes in escherichia coli, so as to obtain recombinant escherichia coli; and carrying out asymmetric reduction reaction in a water phase by taking a resting cell of the recombinant escherichia coli as a whole-cell biocatalyst, butanedione as a substrate and formic acid or formate as a coenzyme NADH regenerated hydrogen donor, so as to generate chiral (S)-acetoin. A whole coenzyme regeneration system of the cell is utilized, and only a proper amount of formic acid or formate needs to be added as the hydrogen donor, so that the transfer ability of a thallus is improved; expensive coenzyme NADH does not need to be added, and the yield of chiral (S)-acetoin can reach up to 46.1g / L; the process is simple and efficient, the production cost is low, and the problems of low efficiency and high cost of traditional conversion are solved; and the method has an extremely high economic value and is applicable to large-scale business application.
Owner:GUANGXI ACAD OF SCI
Method for preparing chiral 3R, 5S-dihydroxyl compound by nonaqueous phase
ActiveCN101880694AHigh substrate concentrationPractical application value is greatFermentationOrganic solventOscillatory reaction
Owner:金隆科技集团有限公司
Synthesis of (R)-styrene glycol by coupling acceleration of (R)-carbonyl reduction enzyme and formic dehydrogenase
ActiveCN101469318AHigh optical purityHigh yieldBacteriaMicroorganism based processesEscherichia coliHigh concentration
The invention relates to promotion of synthesis of (R)-styrene glycol by coupling (R)-carbonyl reductase and formic dehydrogenase, which belongs to the technical field of asymmetrical transformation of biological catalysis. The invention provides a recombinant Escherichia coli, namely E.coli Rosetta / pETDuet-rcr-fdh, with a preservation number of CCTCC NO: M208123, wherein the (R)-special carbonyl reductasei and the formic dehydrogenase are coupled by a co-expression mode; asymmetrical transformation reaction is catalyzed by utilizing a cultured recombinant strain and taking 2-hydroxyacetophenone as a substrate; and the optical purity of the (R)-styrene glycol reaches 100 percent e.e., and the yield of the (R)-styrene glycol reaches 85.9 percent by optimizing the biotransformation reaction conditions. The invention utilizes double-enzyme coupling technology to solve the problem of limitation of regeneration cycle of coenzyme under the condition of high-concentration substrate, provides an effective path for high-efficiency and low-cost preparation of the (R)-styrene glycol, and lays a foundation for industrial application of the biosynthesized (R)-styrene glycol.
Owner:JIANGNAN UNIV
Coenzyme regeneration system and establishment method thereof
ActiveCN105132487ACoenzyme regeneration efficiency is highImprove regeneration efficiencyFermentationVector-based foreign material introductionHeterologousBiochemistry
The invention provides a coenzyme regeneration system which comprises formate dehydrogenase (FDH), leucine dehydrogenase (LDH) and ammonium formate. The invention further provides an establishment method of the coenzyme regeneration system. The establishment method mainly includes the steps of 1), heterogeneous expression of formate dhydrogenase and leucine dehydrogenase and 2), establishment of the coenzyme regeneration system. The coenzyme regeneration system is high in coenzyme regeneration efficiency and suitable for large-scale industrialized application, the establishment method is simple and convenient, and cost of coenzyme reaction is lowered greatly.
Owner:ABA CHEM CORP
Hollow fibrous membrane reactor integrating gas distribution and enzyme catalysis and application of hollow fibrous membrane reactor
InactiveCN105505770AAffects gas distribution performanceEasy to control separatelyBioreactor/fermenter combinationsBiological substance pretreatmentsGas phaseRetention time
The invention relates to a hollow fibrous membrane reactor integrating a gas distribution function and an enzyme catalysis function and an application method of the reactor. A hollow fibrous membrane is used as a gas distributor, the hollow fibrous membrane for distributing the gas and a hollow fibrous membrane of an immobilized enzyme are assembled into a same component, the bottom end of the membrane component and the bottom ends of all fibrous membranes in the component are sealed, the top end of the membrane component and the top end of the hollow fibrous membrane of the immobilized enzyme are sealed, and the top end of the hollow fibrous membrane for distributing the gas is open and is used for introducing CO2 gas; the hollow fibrous membrane reactor is applied to a formate dehydrogenase (FDH) catalytic CO2 methanoic acid synthetic system, by controlling the upstream gas rate, the number of the hollow fibrous membrane for distributing the gas and the micro-structural parameters of the membrane, the quantity and the size of bubbles, the retention time and space distribution in a solution can be adjusted, so that the gas-liquid contact time and the gas-liquid contact area can be increased, the liquid phase can be effectively disturbed, the rapid and efficient dispersion and mixing between the gas phase and the liquid phase and inside the liquid phase can be realized, and the mass transfer effect can be improved.
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Method for preparing L-homophenylalanine
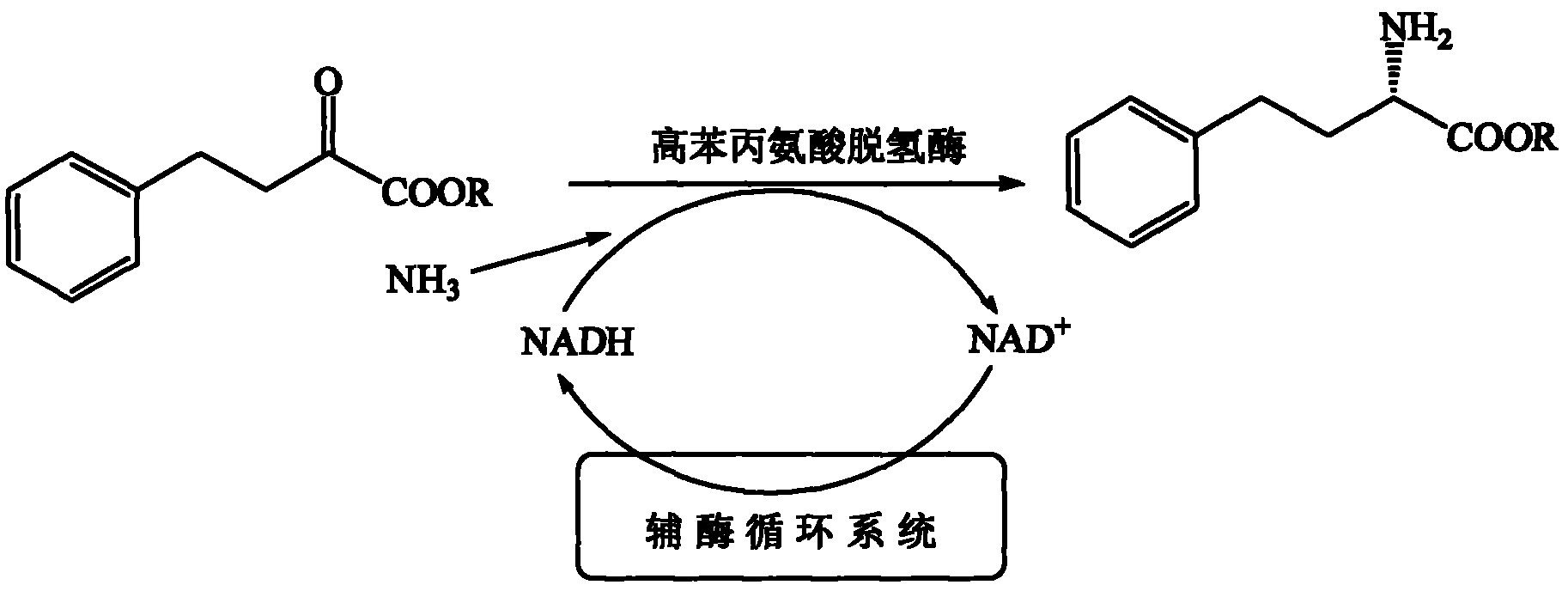
InactiveCN102373246AAchieve regenerationRealize repeated useChemical recyclingFermentationPhenylalanine dehydrogenaseReaction temperature
The invention discloses a process method for preparing single optical pure L-homophenylalanine by using L-homophenylalanine dehydrogenase as a biological catalyst. The method particularly comprises the following steps of: adding a substrate, ammonium formate, the L-homophenylalanine dehydrogenase and a formate dehydrogenase mediated coenzyme recycling and regenerating system into reaction liquid; after reacting under an oscillating or stirring condition, filtering from the reaction system to directly obtain an L-homophenylalanine product; and circulating the filtrate for the next reaction, wherein the reaction temperature is between 15 and 40 DEG C. The L-homophenylalanine is prepared by coordinating the L-homophenylalanine dehydrogenase with the coenzyme recycling and regenerating system and by adopting a simple filtering circulation process, wherein the substrate concentration reaches 560 mM; the NAD+ concentration of the required coenzyme is low; the coenzyme can be recycled and reused for many times, so that the cost of the coenzyme can be ignored; the yield excesses 95 percent; and important application value is achieved.
Owner:陈依军
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com