Method for preparing L-homophenylalanine
A technology of high phenylalanine and high phenylalanine dehydrogenase, applied in chemical recovery, fermentation and other directions, can solve the problems of complex operation of membrane reactor, harsh reaction conditions, complicated reaction process, etc. The effect of low cost and cost reduction of coenzyme
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
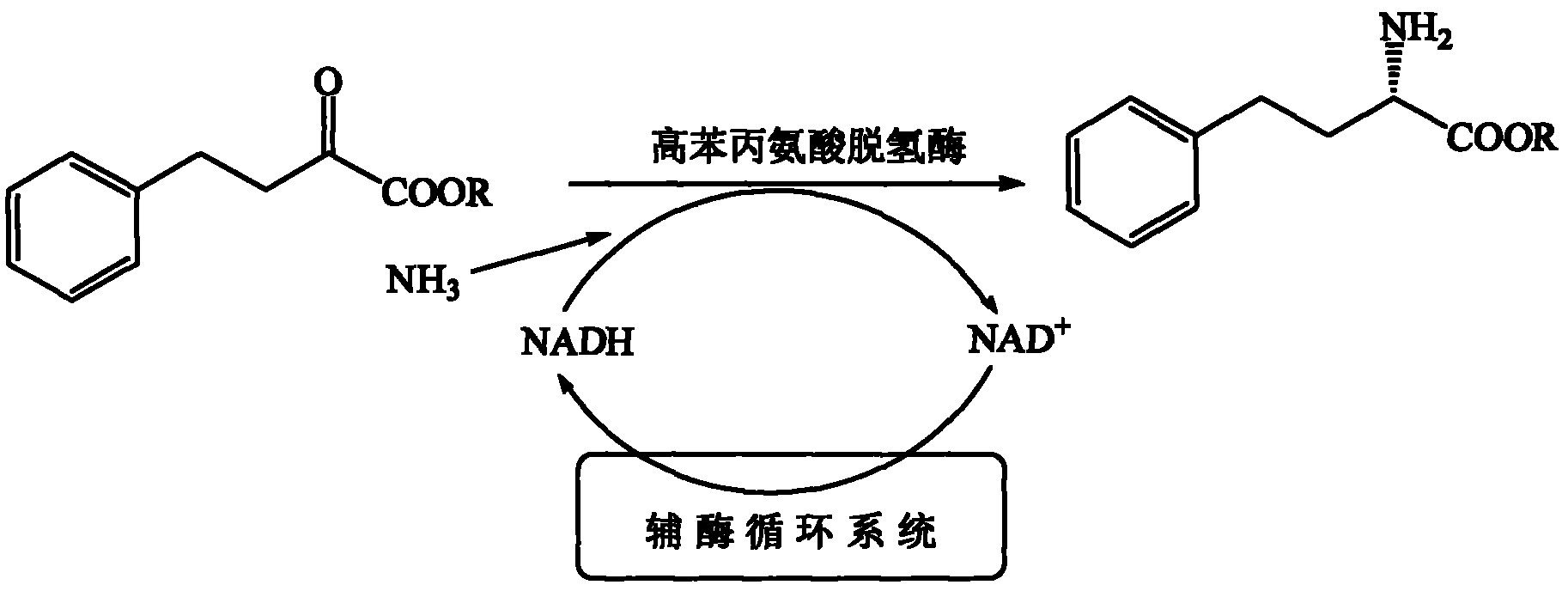
[0036] A method for preparing L-homophenylalanine, the schematic diagram of the preparation process is shown in figure 1 , including the following steps: in a 5mL reaction system, sequentially add substrate 2-oxo-4-phenylbutyric acid, ammonium formate, phosphate buffer, NAD + , homophenylalanine dehydrogenase, formate dehydrogenase, the reaction system contains: substrate 2-oxo-4-phenylbutyric acid 2g / L (11mM), ammonium formate 11~110mM, 0.1M ( pH 8.0) phosphate buffer, NAD + 0.5mM, homophenylalanine dehydrogenase 5U / mL, formate dehydrogenase 5.6U / ml, maintain pH with concentrated potassium hydroxide and phosphoric acid, stir at room temperature for 15-20 hours, after the reaction, filter to obtain L- High phenylalanine, substrate conversion and product optical purity were analyzed by HPLC. The substrate conversion rate was 99.9%.
[0037] Described homophenylalanine dehydrogenase and formate dehydrogenase can be obtained by the following method: the escherichia coli of the...
Embodiment 2
[0040] A method for preparing L-homophenylalanine, comprising the steps of: preparing a 1mL reaction system, said reaction system comprising: substrate ethyl 2-oxo-4-phenylbutyrate 5g / L (25mM, 50μl ethanol for dissolution), ammonium formate 25~250mM, 0.1M (pH 8.5) phosphate buffer, NAD + 0.5mM, homophenylalanine dehydrogenase 5U / mL, formate dehydrogenase 5.6U / ml, shake at room temperature for 20 hours. After the reaction was completed, L-homophenylalanine was obtained by filtration, and the conversion rate of the substrate and the optical purity of the product were analyzed by high performance liquid chromatography. The substrate conversion rate was 99.9%.
Embodiment 3
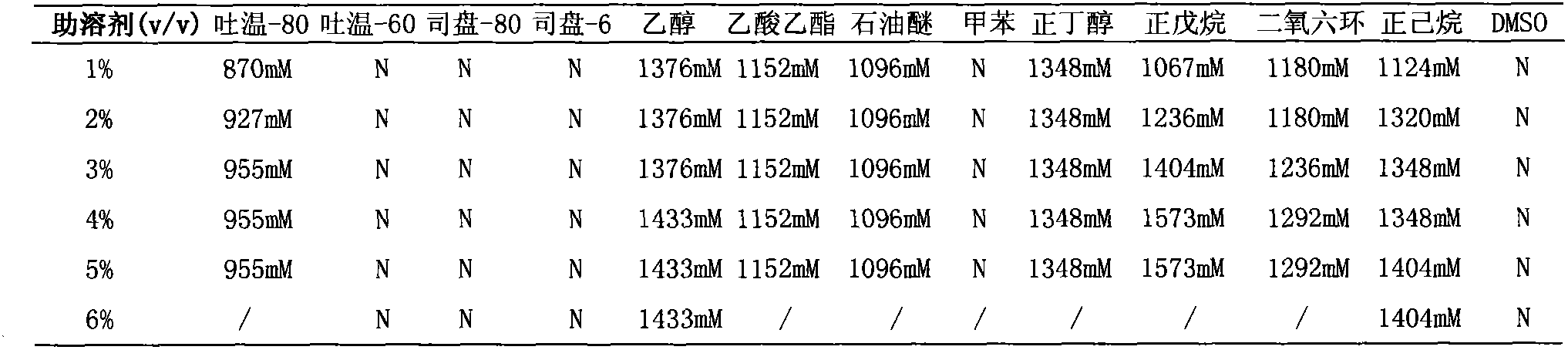
[0042] The preparation of the substrate stock solution includes the following steps: dissolving the ammonium formate and the substrate in a buffer solution or water with a pH value of 7.0 to 9.0 at a molar ratio of 1:1 to 10:1, dissolving at room temperature or slightly heating, and maintaining the substrate with an alkaline solution. The pH of the solution is 7.0-9.0, and a mixed solution of the substrate and ammonium formate is prepared, and the concentration of the substrate is less than or equal to 850mM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com