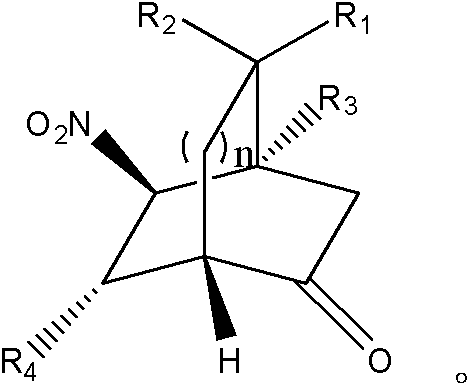
Chiral dicyclic compound and asymmetric syntheses method thereof
A synthesis method and compound technology, which are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of restricting the rapid development of chiral bicyclic diene compounds, and achieve good reaction characteristics and simple reaction operations. , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Example 1: (1S, 4S, 5S, 6R) 6-(2-methoxystyryl)-5-nitrobicyclo[2,2,2]octan-2-one
[0074] Add 2-methoxystyryl nitroalkene (0.102g, 0.5mmol), cyclohexenone (0.192g, 2mmol) and 1mL chloroform in the 25mL test tube, in the chiral secondary amine catalyst (IV) (0.02g, 0.1mmol), PPG1000 (0.3g, 0.3mmol) and benzoic acid (0.012g, 0.1mmol) were jointly catalyzed and reacted at 25°C for 36h, extracted with ethyl acetate (3×20mL), and after distillation to remove the solvent, use Diethyl ether-petroleum ether system is used as eluent (diethyl ether volume content is 20%), 100-200 mesh column chromatography silica gel is used as filler, column chromatography is separated and purified to obtain the target compound (0.102g, yield 68%, appearance / Endotype (exo / endo) > 20:1, Ee value is 90%), where 1 H NMR (500MHz, CDCl 3 ): δ=7.334-7.309 (m, 1H), 7.234-7.207 (m, 1H), 6.906-6.813 (m, 2H), 6.476-6.444 (m, 1H), 6.014-5.944 (m, 1H), 4.622 -4.613(m, 1H), 3.829(s, 3H), 3.750-3.703(m, ...
Embodiment 2
[0075] Example 2: Preparation of (1S, 4S, 5S, 6R) 6-(4-methoxystyryl)-5-nitrobicyclo[2,2,2]octan-2-one
[0076] Add 4-methoxystyryl nitroalkene (0.102g, 0.5mmol), cyclohexenone (0.192g, 2mmol) and 1mL chloroform in a 25mL test tube, in the chiral secondary amine catalyst (IV) (0.02g, 0.1mmol), PPG1000 (0.3g, 0.3mmol) and benzoic acid (0.012g, 0.1mmol) were jointly catalyzed and reacted at 25°C for 36h, extracted with ethyl acetate (3×20mL), and after distillation to remove the solvent, use Diethyl ether-petroleum ether system is used as eluent (diethyl ether volume content is 20%), 100-200 mesh column chromatography silica gel is used as filler, and column chromatography is separated and purified to obtain the target compound (0.123g, yield 66%, appearance / Endotype (exo / endo) > 20:1, Ee value 94%), where 1 H NMR (500MHz, CDCl 3 ): δ=7.261-7.238 (m, 2H), 6.837-6.820 (m, 2H), 6.462-6.430 (m, 1H), 5.842-5.795 (m, 1H), 4.564-4.554 (m, 1H), 3.791 (s, 3H), 3.697-3.671(m, 1H), 2...
Embodiment 3
[0077] Example 3: Preparation of (1S, 4S, 5S, 6R) 6-(4-methylstyryl)-5-nitrobicyclo[2,2,2]octan-2-one
[0078] Add 4-methylstyryl nitroalkene (0.094g, 0.5mmol), cyclohexenone (0.192g, 2mmol) and 1mL chloroform in the 25mL test tube, in chiral secondary amine catalyst (IV) (0.02g, 0.1 mmol), PPG1000 (0.3g, 0.3mmol) and benzoic acid (0.012g, 0.1mmol) under the co-catalysis of 25 ℃ for 36h, extracted with ethyl acetate (3 × 20mL), after distilling off the solvent, using diethyl ether -petroleum ether system is the eluent (ether volume content is 20%), 100-200 mesh column chromatography silica gel is used as filler, column chromatography is separated and purified to obtain the target compound (0.090g, yield 64%, exo / internal Type (exo / endo) > 20:1, Ee value is 86%), where 1 H NMR (500MHz, CDCl 3 ): δ=7.213-7.197(d, J=8Hz, 2H), 7.108-7.092(d, J=8Hz, 2H), 6.488-6.456(d, J=16Hz, 1H), 5.933-5.886(m, 1H ), 4.566-4.555(m, 1H), 3.710-3.684(m, 1H), 2.945-2.928(m, 1H), 2.568-2.551(m, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com