Chiral gamma, gamma-disubstituted butenolide compound and preparation method thereof
A butenolide and compound technology, applied in the field of chiral γ, can solve the problems of large steric hindrance of substituents and limitation of product structure diversity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] Chiral thiourea bifunctional catalysts I-VII of the present invention:
[0026]
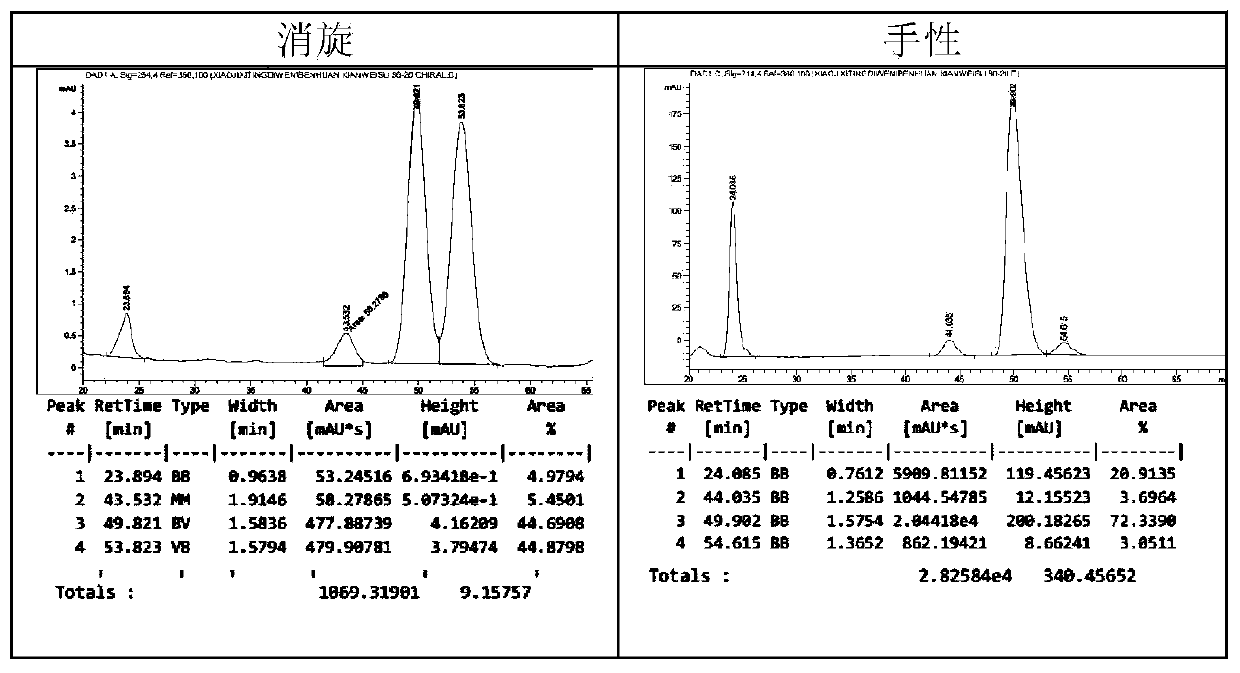
[0027] 1. Compound 3aa: Take compound 1a (0.25mmol) and chiral thiourea bifunctional catalyst VII (0.05mmol), add solvent dioxane (2mL), add compound 2a (0.50mmol), and stir to react. After the reaction was completed, it was quenched with saturated ammonium chloride solution, extracted with ethyl acetate, filtered with suction, and spin-dried. The eluent was ethyl acetate:petroleum ether=1:5 for separation and purification, and spin-dried to obtain compound 3aa.
[0028]
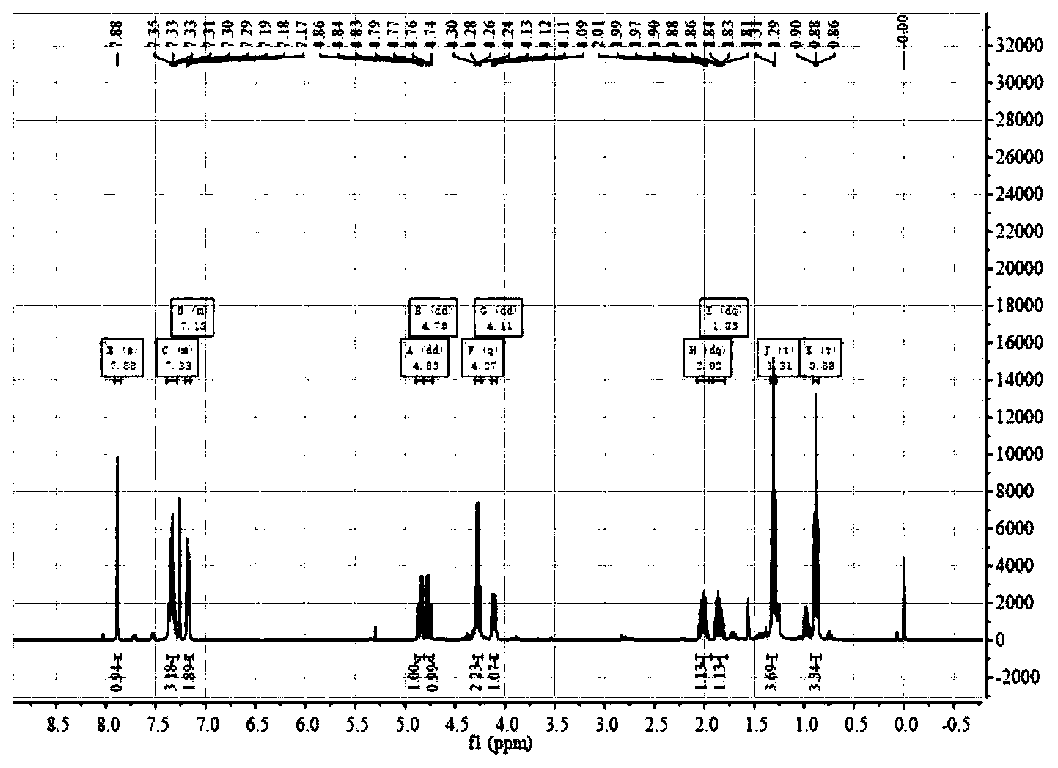
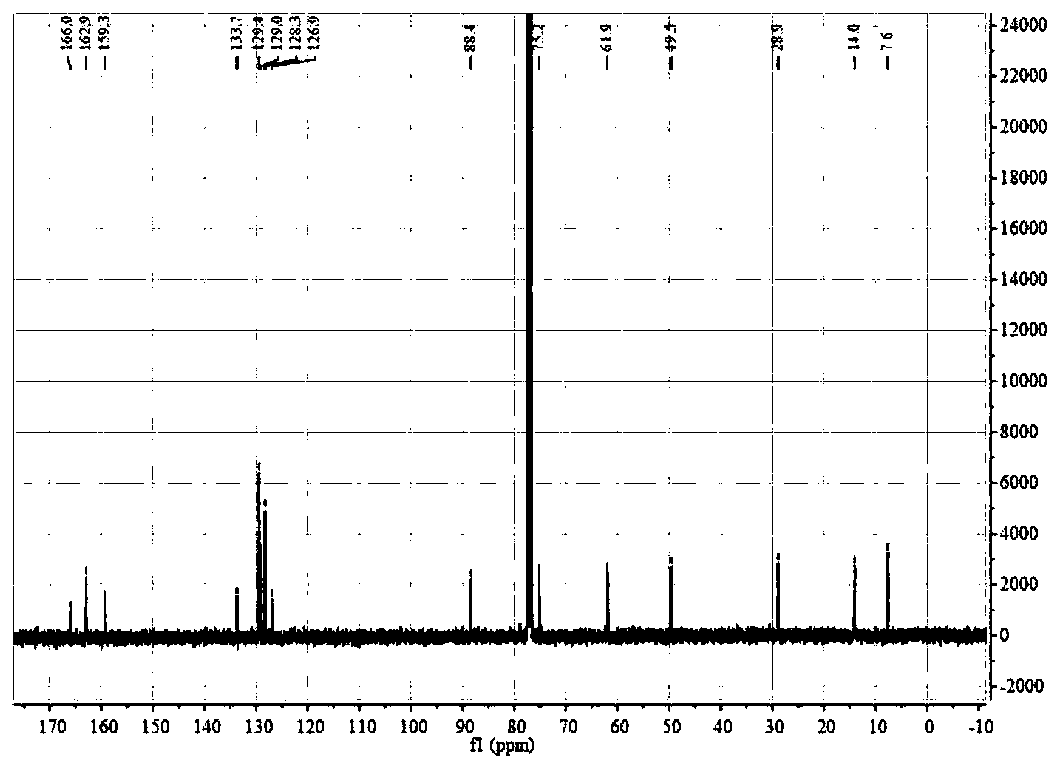
[0029] Yield: 95%. Silica gel column chromatography (ethyl acetate / petroleum ether=1:5); 1 H NMR (400MHz, CDCl 3 ) δ7.88(1H,s), δ7.39–7.28(3H,m), δ7.20–7.15(2H,m), δ4.85(1H,dd, J=13.4,5.0Hz), δ4. 76 (1H, dd, J = 13.3, 10.0Hz), δ4.27 (2H, q, J = 7.1Hz), δ 4.11 (1H, dd, J = 9.9, 5.0Hz), δ2.02 (1H, dq ,J=14.8,7.4Hz),δ1.85(1H,dq, J=14.7,7.4Hz),δ1.31(3H,t,J=7.1Hz),δ0.88(3H,t,J=7.4 Hz). 13 C NMR (100MHz, CDCl 3 )δ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com