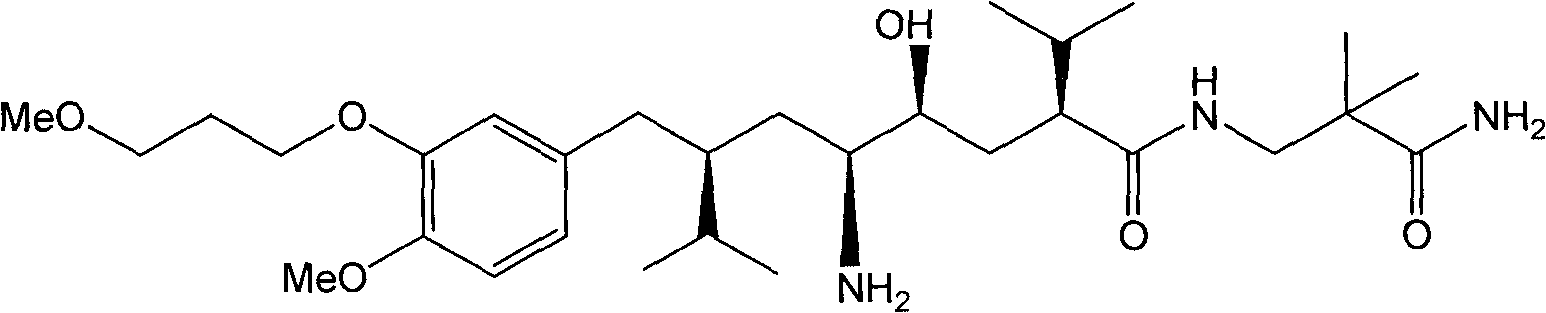
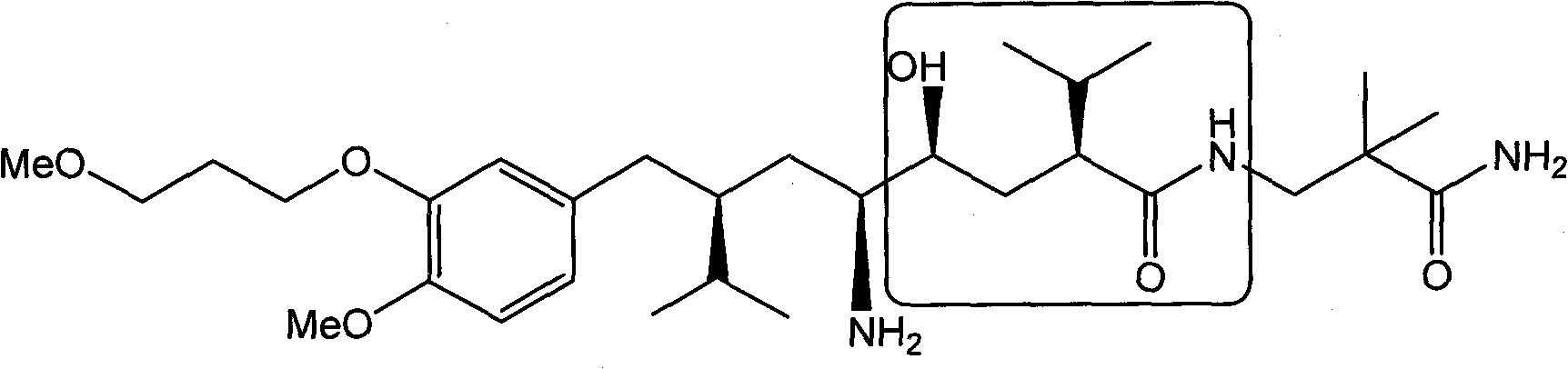
Preparation method of aliskiren intermediate
An intermediate and isopropyl technology, applied in the field of compound preparation, can solve the problems of long synthetic route, production cost and increase of three wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
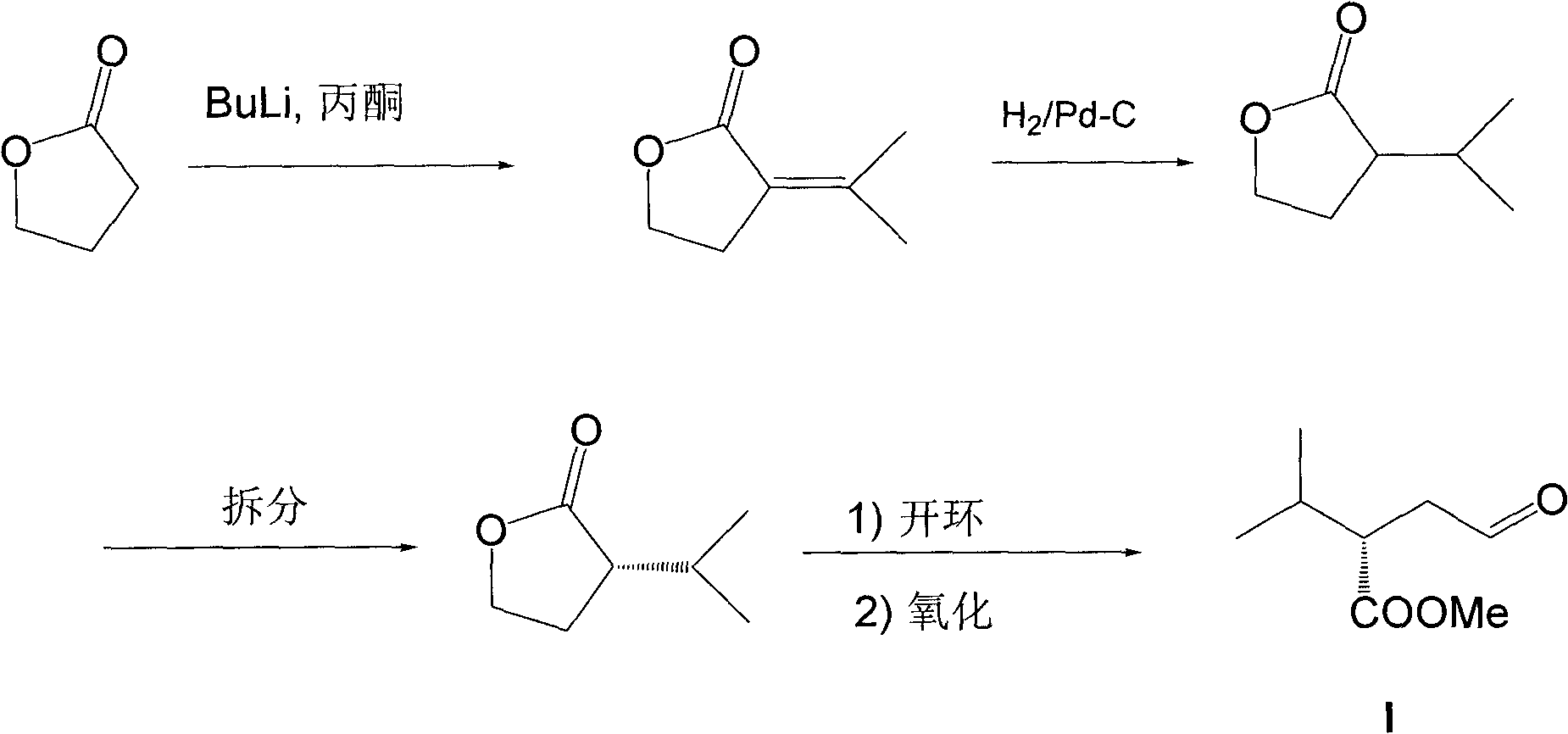
Embodiment 1
[0028] The synthesis of embodiment 1 compound (E)-3-methyl-1-nitrobutene-1
[0029] Add 18.3g (254mmol) of isobutyraldehyde, 15.5g (254mmol) of nitromethane and 50mL of methanol into a 250mL three-necked flask, slowly drop in sodium hydroxide aqueous solution (12.2g of sodium hydroxide dissolved in 12mL of water). Add 10mL of methanol after the addition, and continue to stir the reaction solution for one hour at 0°C, then add 100mL of water to the reaction solution until it becomes clear, and pour the reaction solution into aqueous hydrochloric acid (120mL concentrated hydrochloric acid is diluted with water to 300mL) and stir For 15 minutes, extract three times with dichloromethane, 50 mL each time, combine the organic phases, and wash with anhydrous Na 2 SO 4 Let dry for 2 hours. After filtration, the filtrate was concentrated under reduced pressure and subjected to silica gel column chromatography (petroleum ether (60-90°C) / ethyl acetate 20:1 (v / v)) to obtain light yello...
Embodiment 2
[0030] The preparation of embodiment 2 (S)-4-methyl-3-(nitromethyl)pentanal
[0031] Add catalyst ((S)-diphenyltrimethylsilylmethyl-2-pyrrolidine, 0.65g, 2mmol) in a 25mL single-necked round bottom flask, (E)-3-methyl-1-nitrobutyl En-1 (2.3 g, 20 mmol) and 2 mL of 1,4-dioxane were kept at 4°C, acetaldehyde (8.8 g, 200 mmol) was added, and the mixture was sealed and stirred at room temperature for 18 hours. Then add 10mL1N hydrochloric acid, extract with ethyl acetate (50mL×3), combine the organic phases, wash with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure, and then subjected to silica gel column chromatography (n-hexane / ethyl acetate=20:1 (v / v)) to obtain (S)-4-methyl-3-( Nitromethyl)valeraldehyde 1.8g (11.4mmol), yield 57%.
Embodiment 3
[0032] The preparation of embodiment 3 (S)-2-isopropyl group-4-oxobutanoic acid
[0033] Add (S)-4-methyl-3-(nitromethyl)pentanal (0.6g, 3.8mmol), sodium nitrite (0.79g, 11.4mmol), glacial acetic acid (2.3 g, 38mmol) and 5mL dimethyl sulfoxide, kept the temperature at 35°C and stirred for 6 hours, followed by thin-plate chromatography. Then add 10mL1N hydrochloric acid to the reaction solution, extract with ethyl acetate (30mL×3), combine the organic phases, anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated under reduced pressure, and silica gel column chromatography (dichloromethane / methanol=20:1 (v / v)) gave yellow oil (S)-2-isopropyl-4-oxobutanoic acid 0.4g (2.84mmol), yield 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com