Method for catalyzing asymmetric Michael addition reaction and catalyst
An addition reaction and asymmetric technology, applied in catalytic reactions, physical/chemical process catalysts, organic chemical methods, etc., can solve the problems of low catalytic efficiency, difficult catalyst preparation, poor stability, etc., and achieve high catalytic efficiency and rich variety , the effect of mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
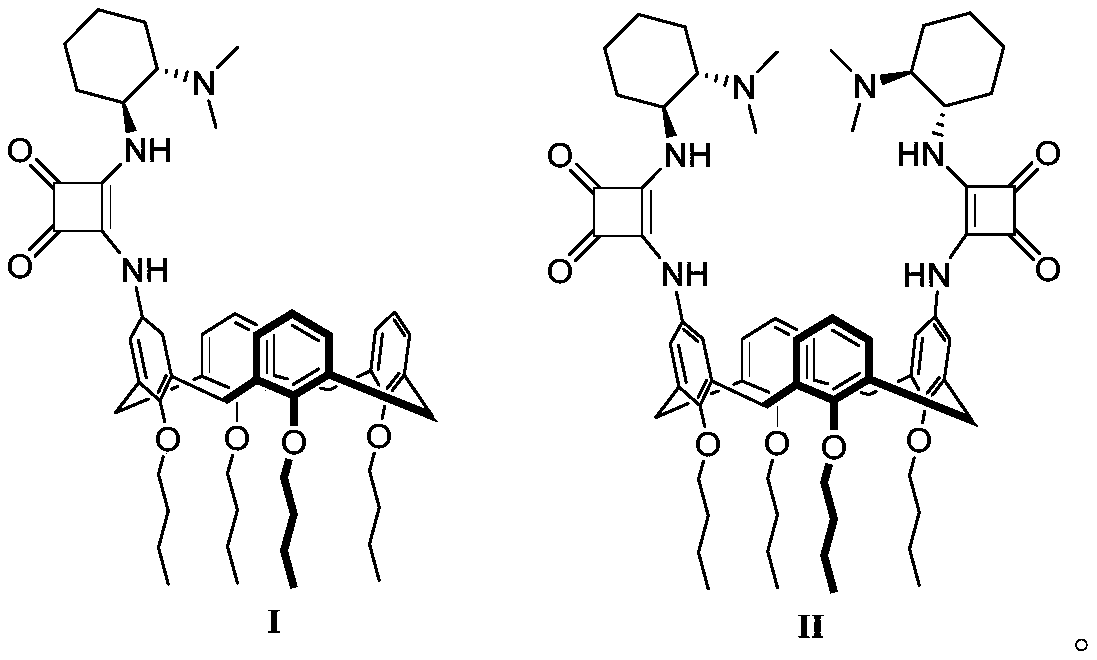
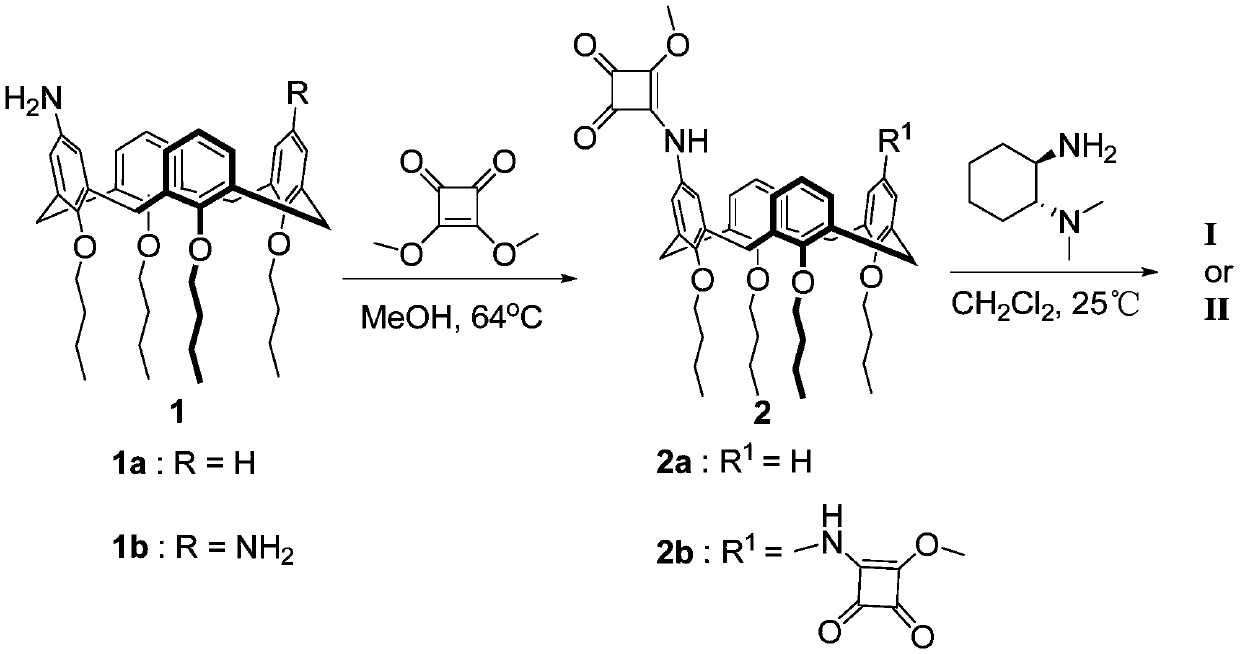
[0026] The present embodiment is the preparation method of calix [4] squaramide cyclohexanediamine catalyst I and II, and the specific synthesis method is as follows:
[0027] Synthesis of Calix[4]Squaramide Cyclohexanediamine Catalysts I and II
[0028]
[0029] Respectively, 1a (0.332g, 0.5mmol) or 1b (0.339g, 0.5mmol), dimethyl squarylate (0.074g, 0.52mmol / 0.149g, 1.05mmol) were heated to reflux in 8mL MeOH solution, followed by TLC spot plate Reaction process, stop the reaction after the disappearance of raw materials, evaporate the solvent, and separate and purify the crude product by column chromatography (ethyl acetate:petroleum ether=1:10) to obtain white solid 2a (0.341g, yield 88%) and light yellow Solid 2b (0.369 g, 82% yield).
[0030] 2a. Mp: 202-204°C; 1 H NMR (300MHz, DMSO-d 6 ):δ=0.98(dt,J 1 =7.2Hz,J 2 =2.1Hz,12H),1.41-1.48(m,8H),1.82-1.90(m,8H),3.15(t,J=12.9Hz,4H),3.81(t,J=6.9Hz,8H),4.31 -4.35(m,4H),4.35(s,3H),6.48-6.66(m,11H),10.44(s,1H). 13 C NMR (...
Embodiment 2
[0036] In this example, using β-nitrostyrene and acetylacetone as substrates, the activity of the asymmetric Michael addition reaction catalyzed by the calix[4]squaramide cyclohexanediamine derivatives I and II was confirmed. The experimental method is: Weigh β-nitrostyrene (0.5mmol, 0.075g), acetylacetone (1mmol, 0.100g) and catalyst (5mol% of β-nitrostyrene) into 1mL dichloromethane In a test tube, stir at 25°C and react for 4 hours. The reaction was stopped, the solvent was concentrated, and separated by column chromatography (ethyl acetate:petroleum ether) to obtain the Michael addition product, and the optical selectivity (ee) of the product was analyzed by HPLC.
[0037] Table 1 The reaction results of β-nitrostyrene and acetylacetone under the catalysis of different catalysts
[0038]
[0039] The results are shown in Table 1. It can be seen that when the calix[4] squaramide cyclohexanediamine derivatives I and II are used as catalysts, the catalyst II with two cata...
Embodiment 3
[0048] In this embodiment, β-nitrostyrene and acetylacetone are used as substrates, and the main influencing factors of the asymmetric Michael addition reaction catalyzed by calix [4] squaramide cyclohexanediamine derivative II are solvent type, reaction temperature, The reaction time and catalyst dosage were systematically studied.
[0049] Result is shown in table 2, 3 and 4, therefore the optimal experimental condition of the asymmetric Michael addition reaction of calix [4] squaramide cyclohexanediamine derivative II catalysis is: catalyst consumption is 5mol%, methylene chloride is used as solvent, the reaction temperature is 25°C.
[0050] Table 2 The influence of temperature on the asymmetric Michael addition reaction catalyzed by calix [4] squaramide cyclohexanediamine derivative II
[0051]
[0052] Table 3 The influence of solvents on the asymmetric Michael addition reaction catalyzed by calix[4]squaramide cyclohexanediamine derivative II
[0053]
[0054] Ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com