Electrophiles and electrophile pro-drugs as rad51 inhibitors
A technology of DNA damage agent and anti-tumor agent, which can be used in the field of electrophilic reagents and electrophilic prodrugs as RAD51 inhibitors, and can solve problems such as harmfulness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
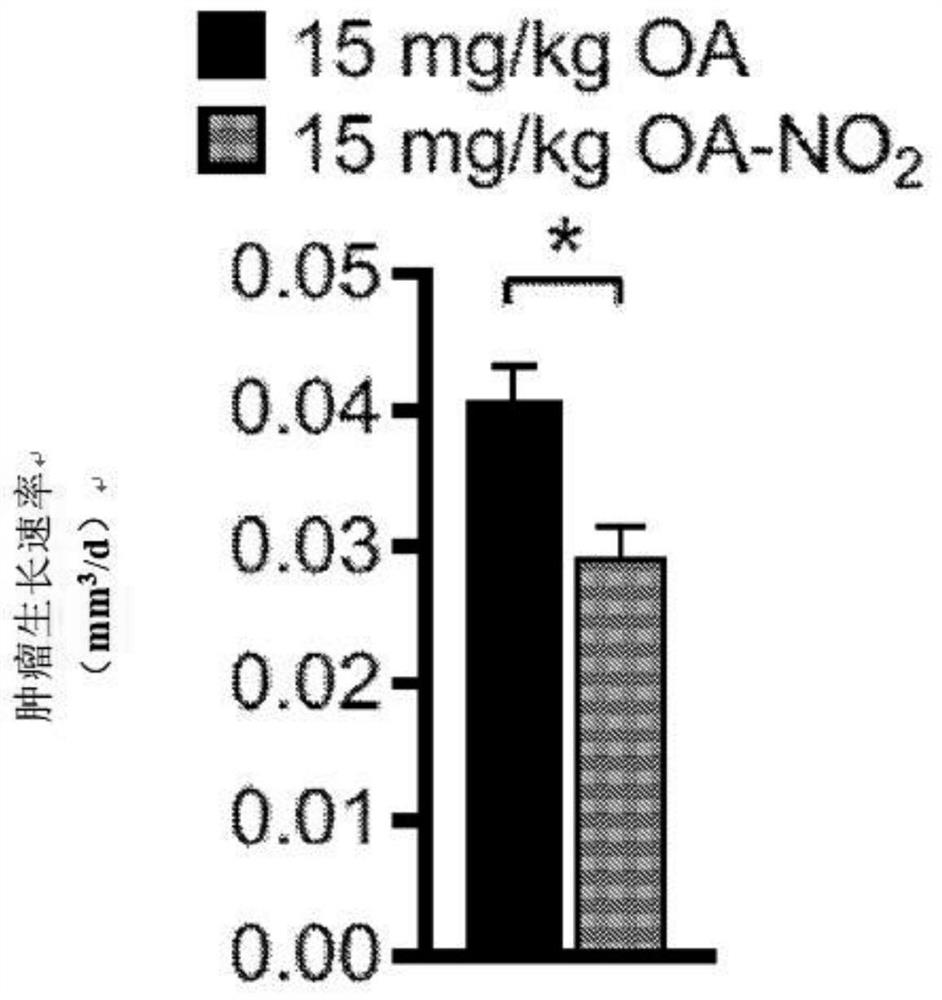
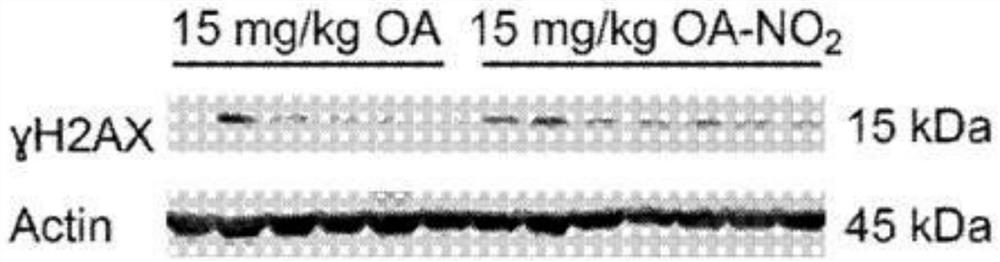
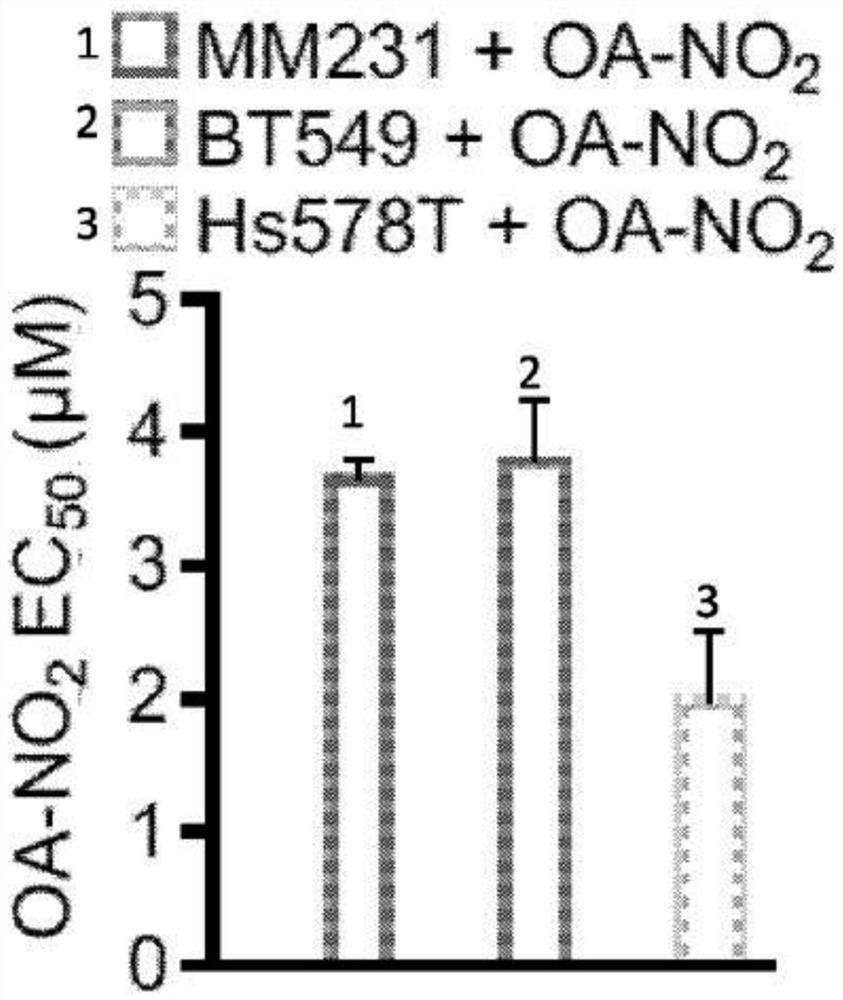
[0277] Experimental procedure - nitroalkene fatty acids impair RAD51 function and enhance the effects of DNA damaging agents on the growth of triple-negative breast cells
[0278] Cell Culture and Reagents
[0279] HEK 293T, MDA-MB-231, MDA-MB-468, Hs578T, and BT-549 cells (American TypeCulture Collection) were incubated at 37°C with 5% CO 2 Cultured in Dulbecco's modified Eagle medium containing 5% FBS (HyClone), 100 units / ml penicillin, 100 mg / ml streptomycin (Gibco), non-essential amino acids (Gibco) and 2 mM 1-glutamine (Gibco). Doxorubicin (Selleckchem), cisplatin (Sigma) or olaparib (Selleckchem) were dissolved in DMSO or DMF (cisplatin). Nitrooleic acid (10-octadec-9-enoic acid) (NO 2 -OA) and biotinylated NO 2 -OA. pure NO 2 -OA was diluted in DMSO and added to cells after solvation in assay medium. The relative number of cells was compared by measuring the luminescence signal produced by ATP by the Cell Titer Glo (Promega) method. Cells were seeded in 96-well p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com