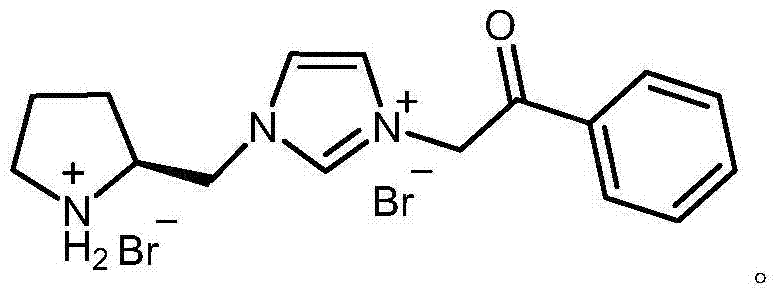
Chiral pyrrolidine functionalized imidazolium salt, and preparation method and application thereof
A pyrrolidine and functionalization technology is applied in the synthesis field of chiral compounds to achieve the effects of high diastereoselectivity and enantioselectivity, readily available raw materials and convenient preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
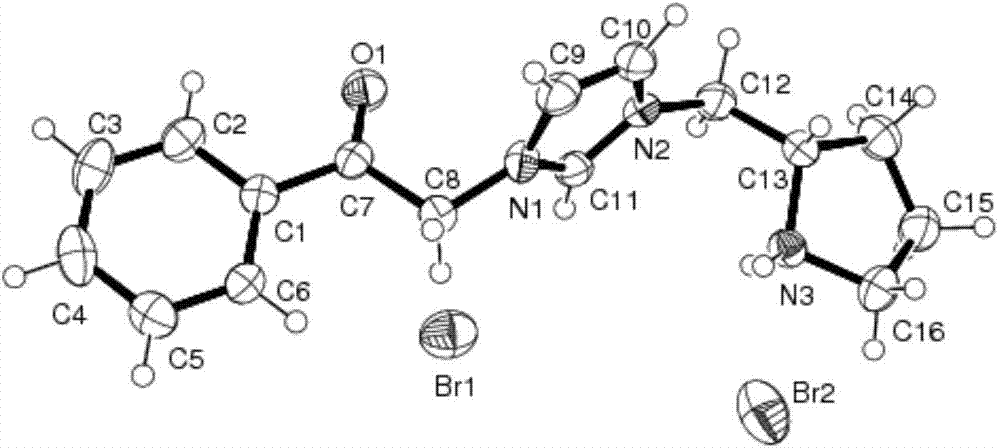
[0025] Preparation of 1-[2-(S)-(pyrrolidinyl)methyl]-3-phenacyl imidazolium bromide hydrobromide
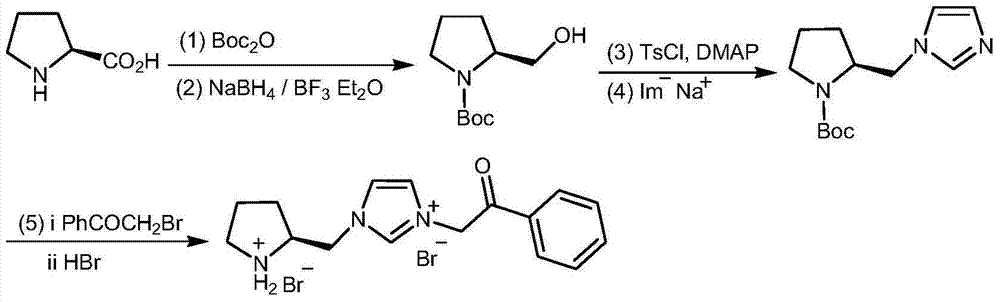
[0026] (1) In a 1L reaction flask, add 23g (0.2mol) L-proline and 400mL dichloromethane, start stirring, cool to 0°C with an ice-water bath, then add 26.3g (0.26mol) triethylamine, Then add 61g (0.28mol) Boc in batches 2 O, control the feed rate to keep the temperature of the reaction system at 0±2°C, and keep the reaction at 0°C for 2.5 hours after the addition is complete. The reaction solution was washed with saturated aqueous citric acid solution, the organic phase was separated, the solvent was removed under reduced pressure, and recrystallized with a mixed solvent of ethyl acetate and petroleum ether to obtain 39.5 g of Boc-L-proline with a yield of 92%.
[0027] (2) In a 500mL reaction flask, add 6.08g (0.16mol) sodium borohydride and 90mL isopropyl acetate, start stirring, cool to -5°C with an ice-water bath, then add 21.5g (0.1mol) Boc-L - Proline, after the addition, ...
Embodiment 2
[0038] Preparation of 1-[2-(S)-(pyrrolidinyl)methyl]-3-phenacyl imidazolium bromide hydrobromide
[0039] (1) The preparation of Boc-L-proline is the same as in Example 1;
[0040] (2) The preparation of Boc-L-prolinol is the same as in Example 1;
[0041] (3) The preparation of (S)-2-(p-toluenesulfonyloxymethyl)pyrrolidine-1-carboxylic acid tert-butyl ester is the same as in Example 1;
[0042] (4) The preparation of (S)-2-(1-imidazolylmethyl)pyrrolidine-1-carboxylic acid tert-butyl ester is the same as in Example 1;
[0043] (5) In a 250mL reaction flask, add 100mL toluene and 5.02g (20mmol) (S)-2-(1-imidazolylmethyl)pyrrolidine-1-carboxylic acid tert-butyl ester, start stirring, then add 4.78g (24 mmol) α-bromoacetophenone, heated to reflux for 10 hours. Cool, remove toluene under reduced pressure, and separate by column to obtain N-Boc-protected chiral pyrrolidine functionalized imidazolium salt. Dissolve it in 80 mL of anhydrous methanol, add 11.7 mL of 40% hydrobromic...
Embodiment 3
[0045] Preparation of 1-[2-(S)-(pyrrolidinyl)methyl]-3-phenacyl imidazolium bromide hydrobromide
[0046] (1) The preparation of Boc-L-proline is the same as in Example 1;
[0047] (2) The preparation of Boc-L-prolinol is the same as in Example 1;
[0048] (3) The preparation of (S)-2-(p-toluenesulfonyloxymethyl)pyrrolidine-1-carboxylic acid tert-butyl ester is the same as in Example 1;
[0049] (4) The preparation of (S)-2-(1-imidazolylmethyl)pyrrolidine-1-carboxylic acid tert-butyl ester is the same as in Example 1;
[0050] (5) In a 250mL reaction flask, add 100mL toluene and 5.02g (20mmol) (S)-2-(1-imidazolylmethyl)pyrrolidine-1-carboxylic acid tert-butyl ester, start stirring, then add 5.97g (30mmol) α-bromoacetophenone, heated to reflux for 12 hours. Cool, remove toluene under reduced pressure, and separate by column to obtain N-Boc-protected chiral pyrrolidine functionalized imidazolium salt, dissolve it in 80 mL of anhydrous methanol, add 14.7 mL of 40% hydrobromic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com