Application of porcine pancreatic lipase as catalyst of asymmetric aldol reaction of heterocyclic ketone and aromatic aldehyde
A technology of porcine pancreatic lipase and heterocyclic ketones, which is applied in the chemical field to achieve the effects of high yield, broad application prospects, and high ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1, Porcine pancreas lipase catalyzed activity confirmation of heterocyclic ketone and aromatic aldehyde asymmetric aldol reaction
[0028] In this example, the reaction of 1-Boc-4-piperidone and p-nitrobenzaldehyde was used as a template reaction to confirm the activity of porcine pancreatic lipase in catalyzing the asymmetric aldol reaction between heterocyclic ketones and aromatic aldehydes. The experimental method is: add 1-Boc-4-piperidone (0.25mmol) and p-nitrobenzaldehyde (0.50mmol) in the reaction flask, do not add catalyst or add catalyst, then add 1mL solvent (divided by sodium bicarbonate The catalyst is ethanol as the solvent, and the rest of the catalysts are added with a water-acetonitrile mixed solvent with a volume ratio of 1:9), and the temperature is controlled at 25°C to stir the reaction; after the reaction is completed, filter, the filter cake is washed with dichloromethane, and the filtrates are combined and washing solution, dilute with w...
Embodiment 2
[0033] Example 2. Methodological research on the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes catalyzed by porcine pancreatic lipase
[0034]In this embodiment, the reaction of 1-Boc-4-piperidone and p-nitrobenzaldehyde is used as a template reaction, and the main influencing factors (solvent, water content) of the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes catalyzed by porcine pancreatic lipase , substrate feed ratio, enzyme amount, reaction temperature, reaction time) were systematically studied.
[0035] 1. The influence of solvent
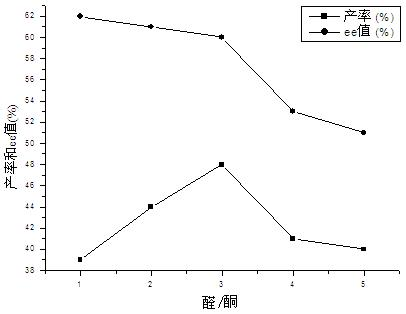
[0036] Add 1-Boc-4-piperidone (0.25mmol), p-nitrobenzaldehyde (0.50mmol) and porcine pancreatic lipase (50mg) into the reaction flask, then add 1mL solvent, and stir the reaction at 25°C for 120 hours , aftertreatment method is the same as embodiment 1. The results are shown in Table 2.
[0037] Table 2 The effect of solvent on the asymmetric aldol reaction catalyzed by porcine panc...
Embodiment 3
[0050] Example 3, General investigation on the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes catalyzed by porcine pancreatic lipase
[0051] This example investigates the versatility of porcine pancreatic lipase in catalyzing the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes. The experimental method is: add heterocyclic ketone (0.25mmol), aromatic aldehyde (0.25mmol) and porcine pancreatic lipase (50mg) into the reaction flask, then add 1mL of aqueous acetonitrile (V water / V acetonitrile = 0.10), control the temperature for 30 ℃ stirring reaction for 120~144 hours, aftertreatment method is the same as embodiment 1. The results are shown in Table 3.
[0052] Table 3 Porcine pancreatic lipase catalyzes the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes
[0053]
[0054]
[0055] It can be seen from Table 3 that the asymmetric aldol reaction of heterocyclic ketones and aromatic aldehydes cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com