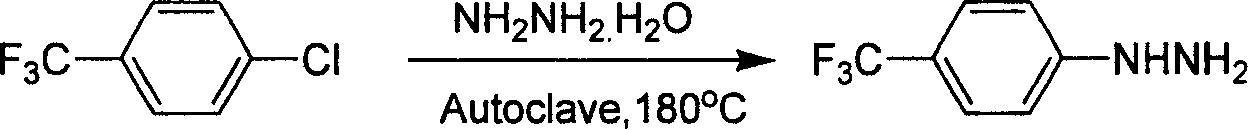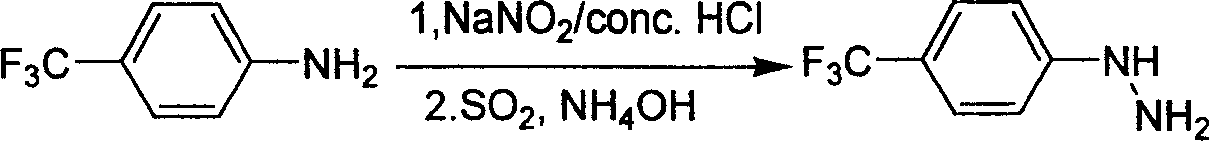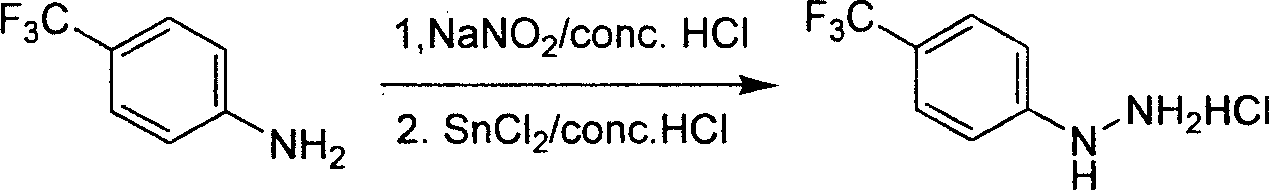Industrial synthesis method for 4-trifluoromethylphenylhydrazine Hydrochloride
A technology of trifluoromethylphenylhydrazine and trifluoromethylaniline, which is applied in the field of industrialized preparation of 4-trifluoromethylphenylhydrazine hydrochloride, can solve the problem that there is no effective industrial synthesis method for preparation, and the yield of literature operation is not high. High, there are safety hazards and other problems, to achieve the effect of low requirements, high overall yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Synthesis of 4-Trifluoromethylphenylhydrazine Hydrochloride
[0030] Under mechanical stirring, a 10-liter three-necked flask equipped with 20% hydrochloric acid aqueous solution (3.5 L) was cooled to 5-10 ° C with an ice bath, and 4-trifluoromethylaniline (500 g, 3.1 mol ). The dropwise addition process is accompanied by heat release, and the reaction solution slowly becomes a suspension. After the dropwise addition, lower the reaction solution to 0-5°C, add sodium nitrite aqueous solution (225g / 0.5L) dropwise to the suspension with a dropping funnel, control the internal temperature below 5°C during the dropping process, and add the time Control in 2 hours. It can be observed that the reaction solution turns into a clear and transparent solution. After stirring for half an hour at 0-5°C, add SnCl dropwise to the clear liquid 2 .2H 2 O (1.7kg, 13.3mol) of hydrochloric acid solution (35-37% HCl, 1.7L), during the dropwise addition, the internal temperature was co...
Embodiment 2
[0032] Synthesis of 4-Trifluoromethylphenylhydrazine Hydrochloride
[0033] Under mechanical stirring, a 10-liter three-neck flask containing 20% hydrochloric acid aqueous solution (3.5 L) was cooled to 0-5 ° C with an ice bath, and 4-trifluoromethylaniline (500 g, 3.1 mol ). The dropwise addition process is accompanied by heat release, and the reaction solution slowly becomes a suspension. After the dropwise addition, lower the reaction solution to 0-5°C, add sodium nitrite aqueous solution (225g / 0.5L) dropwise to the suspension with a dropping funnel, control the internal temperature below 5°C during the dropwise addition, and add the The time is controlled at 2 hours. It can be observed that the reaction solution turns into a clear and transparent solution. After stirring for half an hour at 0-5°C, add SnCl dropwise to the clear liquid 2 .2H 2 O (1.7kg, 13.3mol) of hydrochloric acid solution (35-37% HCl, 1.7L), during the dropwise addition, the internal temperature...
Embodiment 3
[0035] Synthesis of 4-Trifluoromethylphenylhydrazine Hydrochloride
[0036] Under mechanical stirring, a 10-liter three-necked flask equipped with 20% hydrochloric acid aqueous solution (3.5 L) was cooled to 5-10 ° C with an ice bath, and 4-trifluoromethylaniline (500 g, 3.1 mol ). The dropwise addition process is accompanied by heat release, and the reaction solution slowly becomes a suspension. After the dropwise addition, adjust the reaction solution to 5-10°C, add sodium nitrite aqueous solution (225g / 0.5L) dropwise to the suspension with a dropping funnel, control the internal temperature below 5°C during the dropping process, and add the time Control in 2 hours. It can be observed that the reaction solution turns into a clear and transparent solution. After stirring for half an hour at 0-5°C, add SnCl dropwise to the clear liquid 2 .2H 2 O (1.7kg, 13.3mol) of hydrochloric acid solution (35-37% HCl, 1.7L), during the dropwise addition, the internal temperature was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com