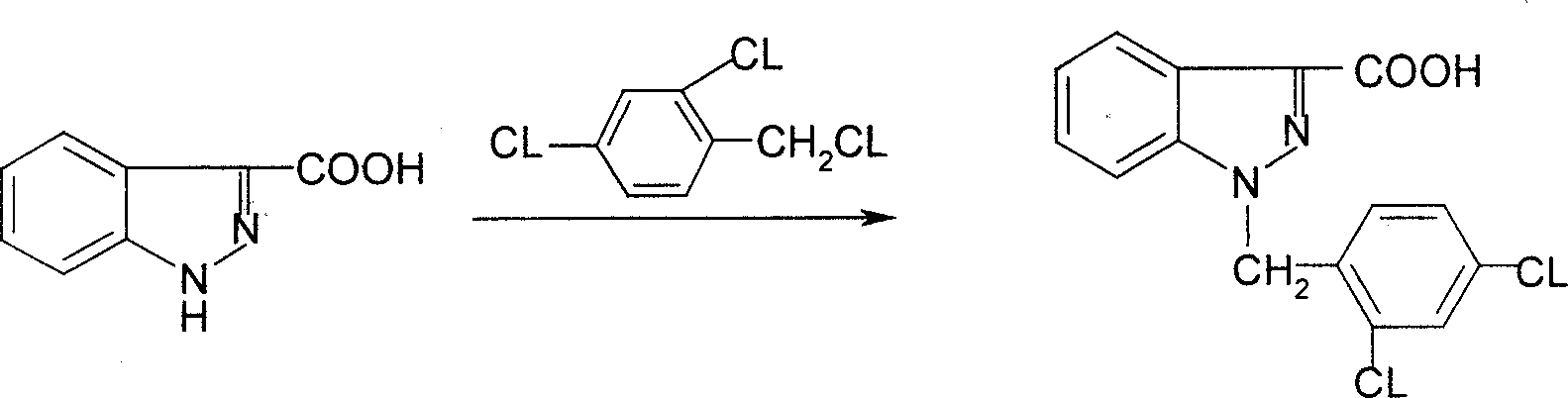
Process for synthesis of lonidamine
A technology of lonidamine and synthetic method, which is applied in the direction of drug combination, organic chemistry, antineoplastic drugs, etc., and can solve the problems of cost and complex production process of lonidamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
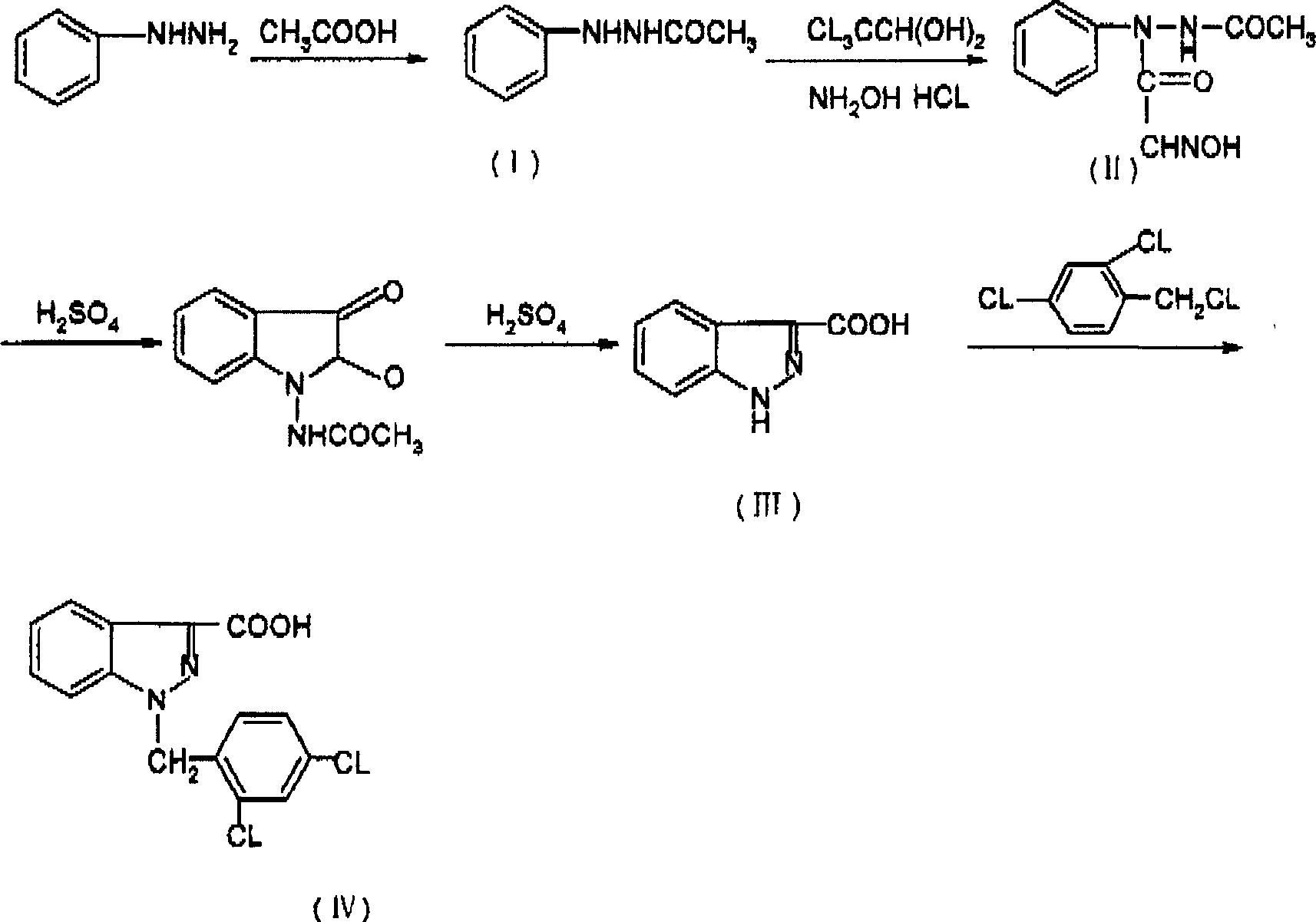
[0029] 1, the synthesis of β-acetylphenylhydrazine (I)
[0030] Add a solution of 265g (2.5mol) of phenylhydrazine, 600ml (10.5mol) of glacial acetic acid and 350ml of water into a 2L three-necked flask. Warm to reflux, stir for 3 hours, then cool to room temperature. The solid was precipitated, filtered, washed with water, and dried to obtain 290 g of off-white crystal I. Yield 78.2%. mp: 127.5-129.5°C
[0031] 2. Synthesis of N-acetylisonitrosoacetanilide (II)
[0032] In the three-necked flask of 3L, add I 76g (0.51mol), water 2300ml, concentrated hydrochloric acid 27ml, hydroxylamine hydrochloride 69 grams (0.9mol), anhydrous sodium sulfate 367 grams, chloral hydrate 157 grams (0.96mol). Raise the temperature to 80--95°C. Keep stirring for 0.4 hours. Rapidly raise the temperature to 110°C, stir for 4 minutes, rapidly cool to 55-65°C, add 5 g of activated carbon for decolorization and stir, quickly filter off the oil, freeze to precipitate a yellow solid, filter, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com