Prepn process of 7-ethyl tryptophol
A technology of ethyl tryptophan and ethyl, which is applied in the field of preparation of 7-ethyl tryptophan, can solve the problems of high production cost, complicated process and low reaction yield, and achieve the advantages of convenient operation, good yield and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
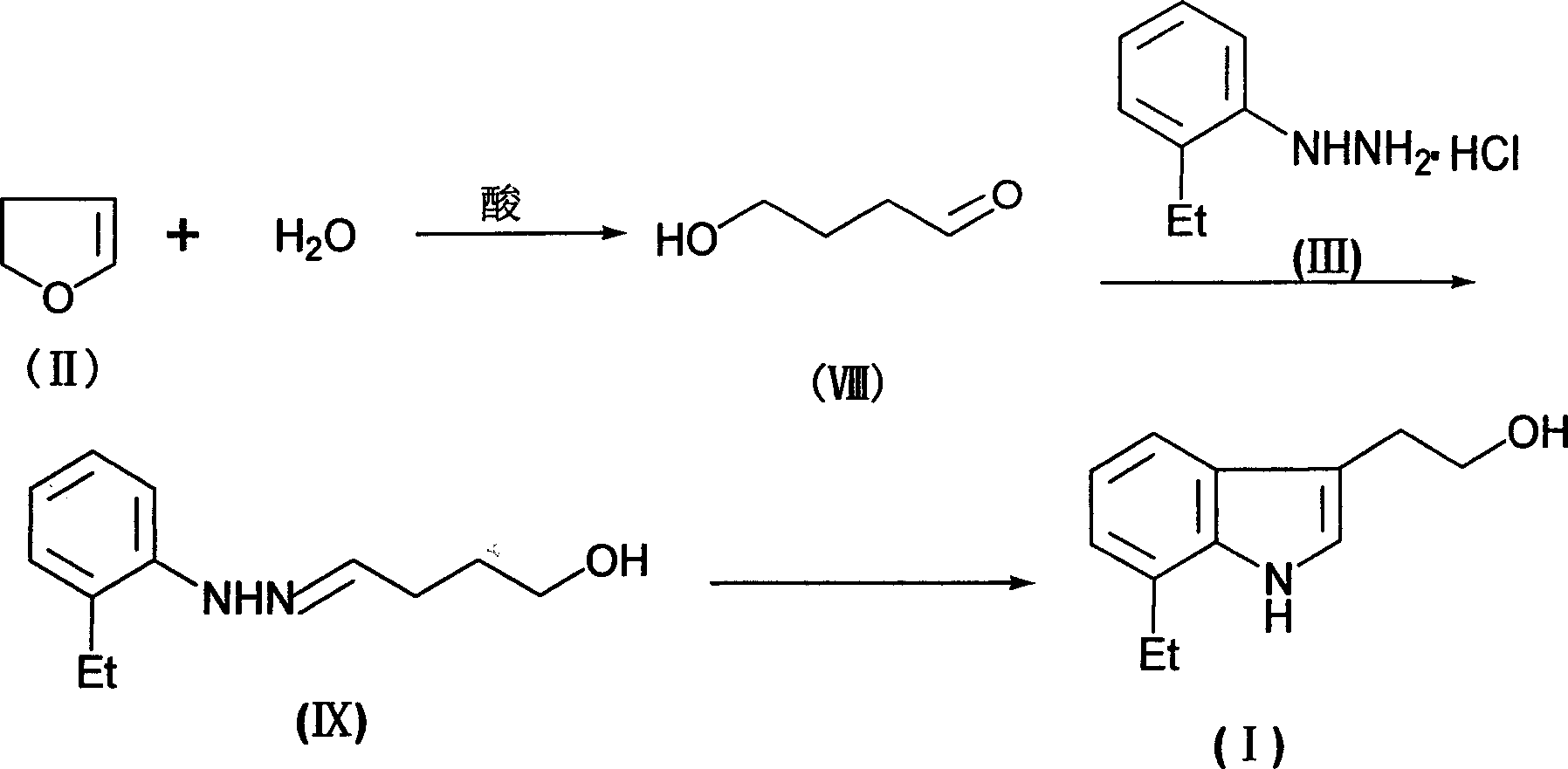
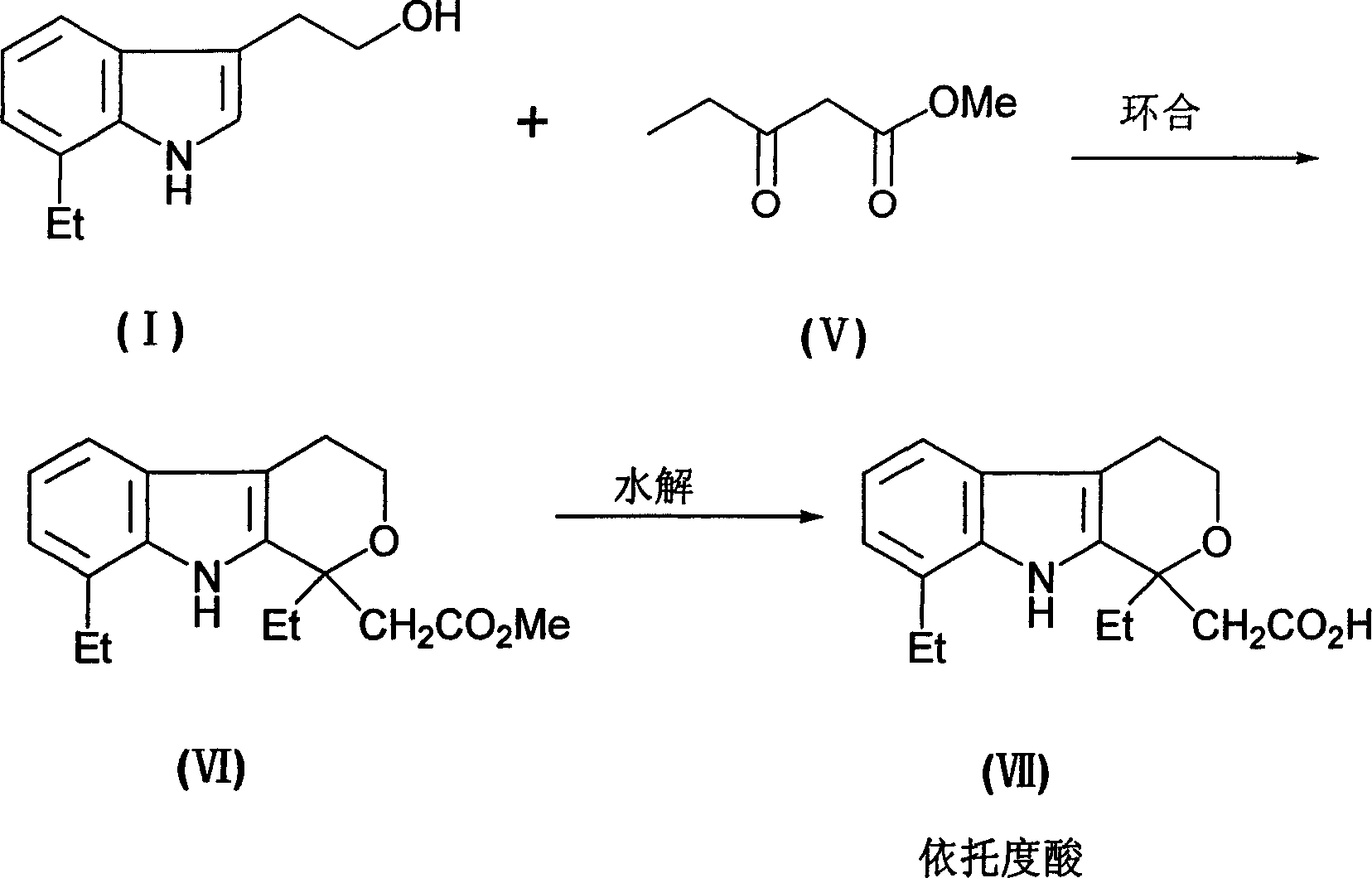
[0021] The preparation of embodiment 1 7-ethyl tryptophan
[0022] In a 500mL reaction flask, add 350g of ethylene glycol monomethyl ether, 18g of water, and 10g of concentrated hydrochloric acid, below 0°C, add 70g of 2,3-dihydrofuran dropwise, and stir for 1 hour after the addition is complete. ,stand-by.
[0023] In another 1500mL reaction flask, add 350 grams of ethylene glycol monomethyl ether and 173 grams of o-ethylphenylhydrazine hydrochloride, and heat to 80°C under stirring. Then, the above-mentioned ethylene glycol monomethyl ether The solution was slowly added dropwise to the reaction solution containing o-ethylphenylhydrazine hydrochloride, after the addition was completed, reacted for 8 hours, concentrated and evaporated to remove ethylene glycol monomethyl ether, added 600 grams of water, 300 grams of dichloromethane, stirred and separated Afterwards, dichloromethane was concentrated, distilled under reduced pressure, and fractions were collected: 180-200°C (5m...
Embodiment 2~12
[0025] The raw material consumption and conditions of the reaction shown in Example 1 were changed, so that the product results obtained are shown in Table 1.
[0026] Example
Embodiment 13-20
[0028] Change the acid that adopts and its consumption, the kind of glycol ether solvent, others are all the same as embodiment 1, and reaction result is as shown in table 2.
[0029]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com