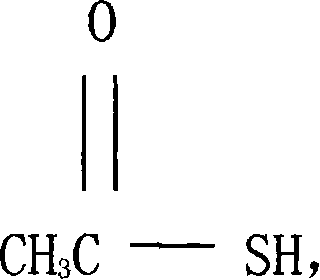Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
119 results about "Thioacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Thioacetic acid is an organosulfur compound with the molecular formula CH₃COSH. It is a yellow coloured liquid with a strong thiol-like odor.
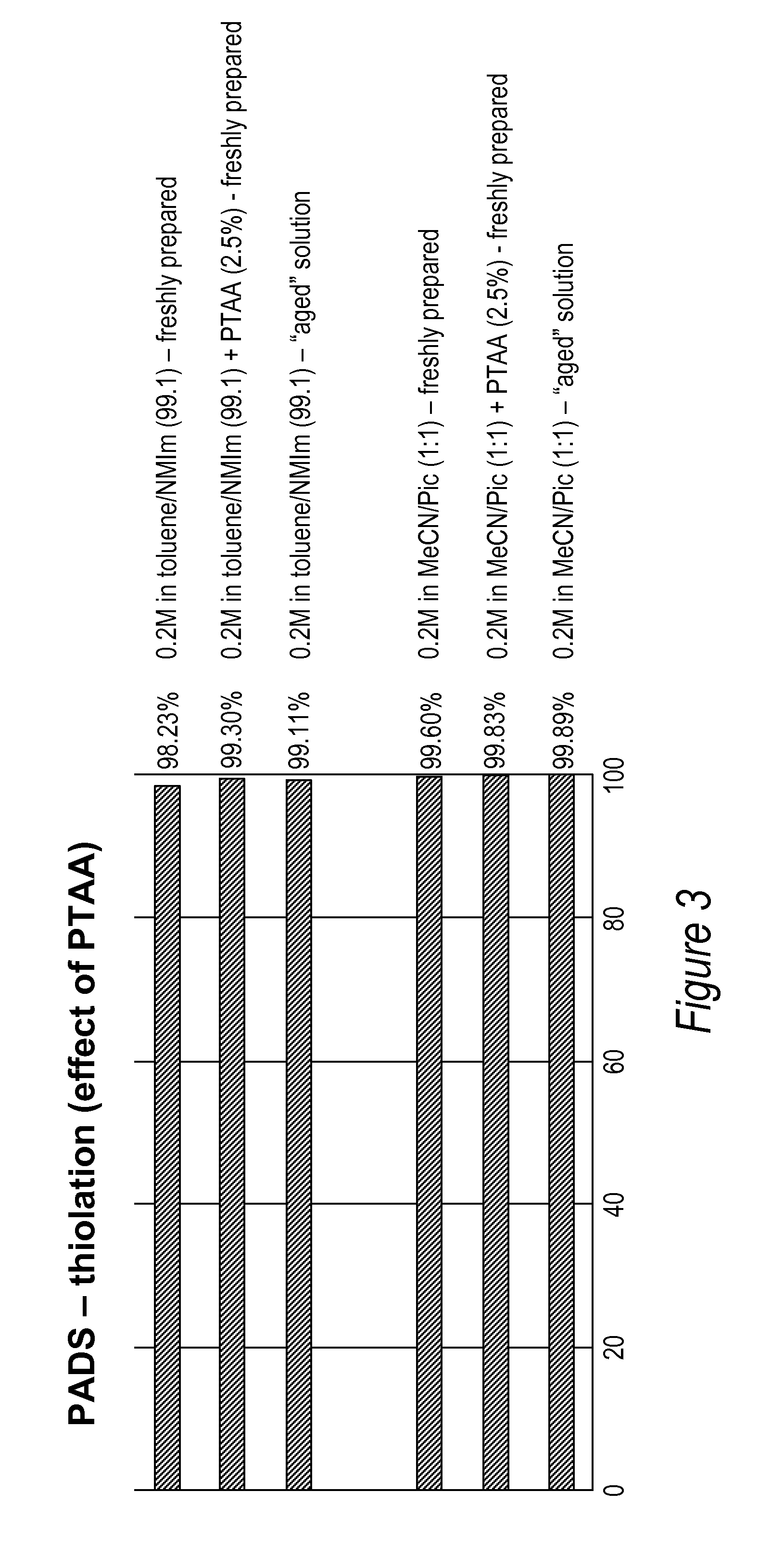
Use of thioacetic acid derivatives in the sulfurization of oligonucleotides with phenylacetyl disulfide
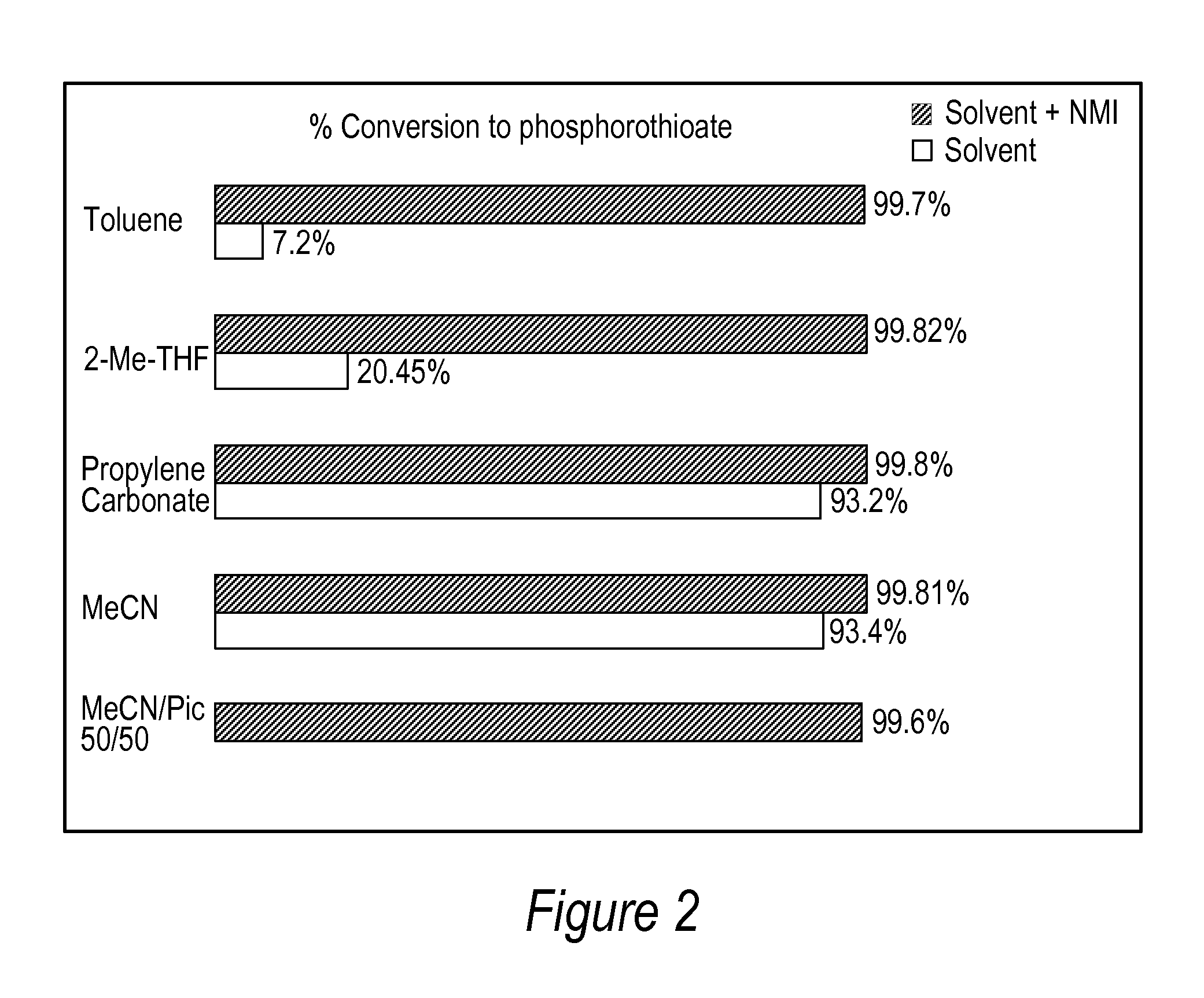
A method and compositions for sulfurizing at least one phosphite or thiophosphite linkage in an oligonucleotide. The methods employ a phenylacetyl disulfide reagent (known as PADS), phenylthioacetic acid (PTAA) in the presence or absence or N-alkyl imidazole in industrially preferred solvents or solvents that are derived from renewable resources. The use of PTAA eliminates the need to “age” the PADS solution prior to its use in sulfurization reactions.
Owner:AGILENT TECH INC
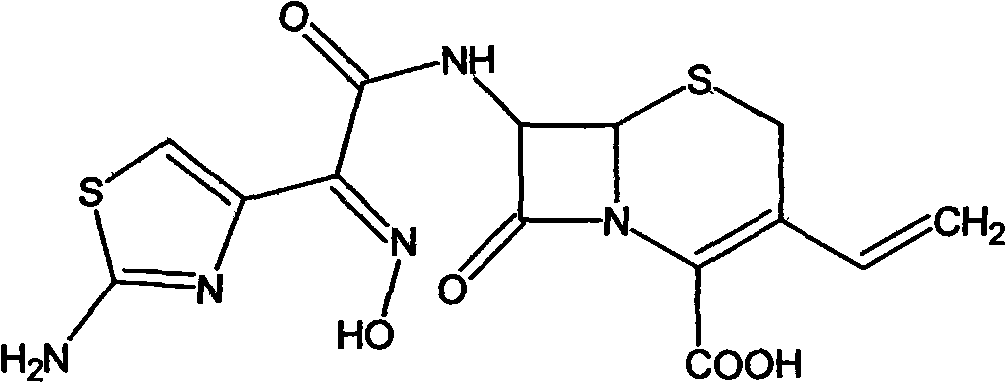
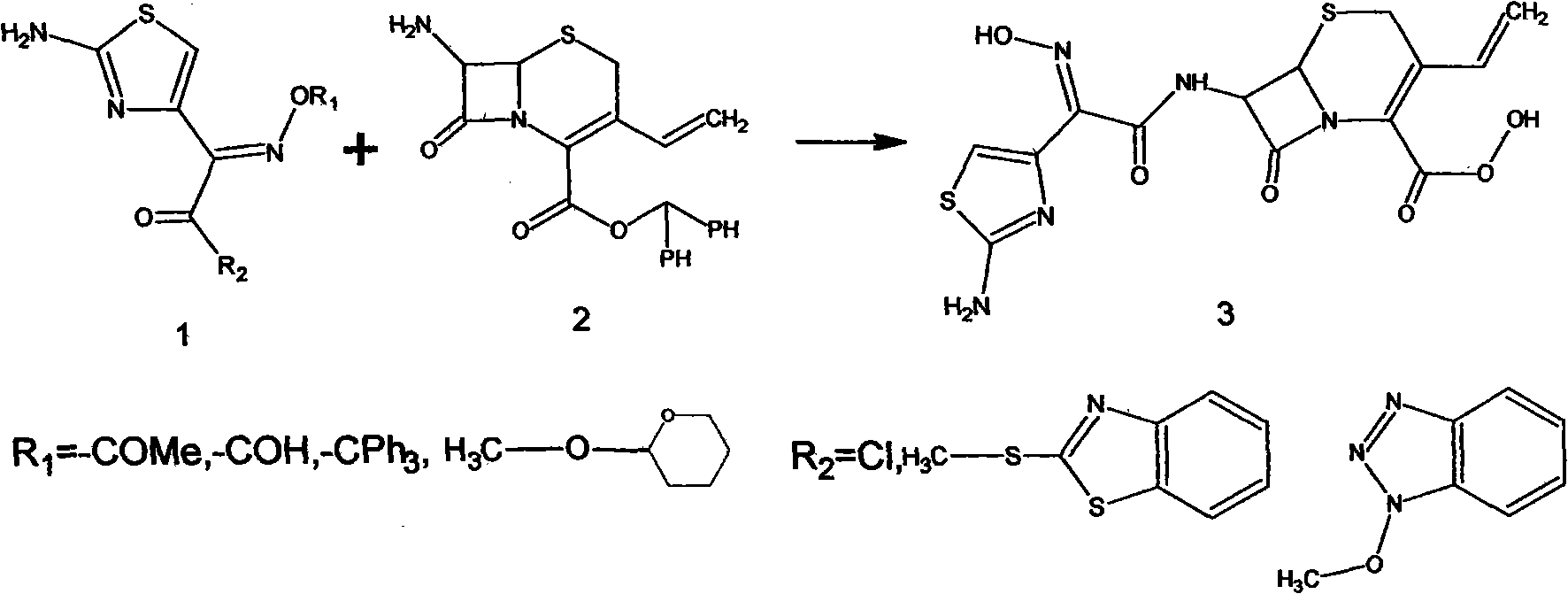
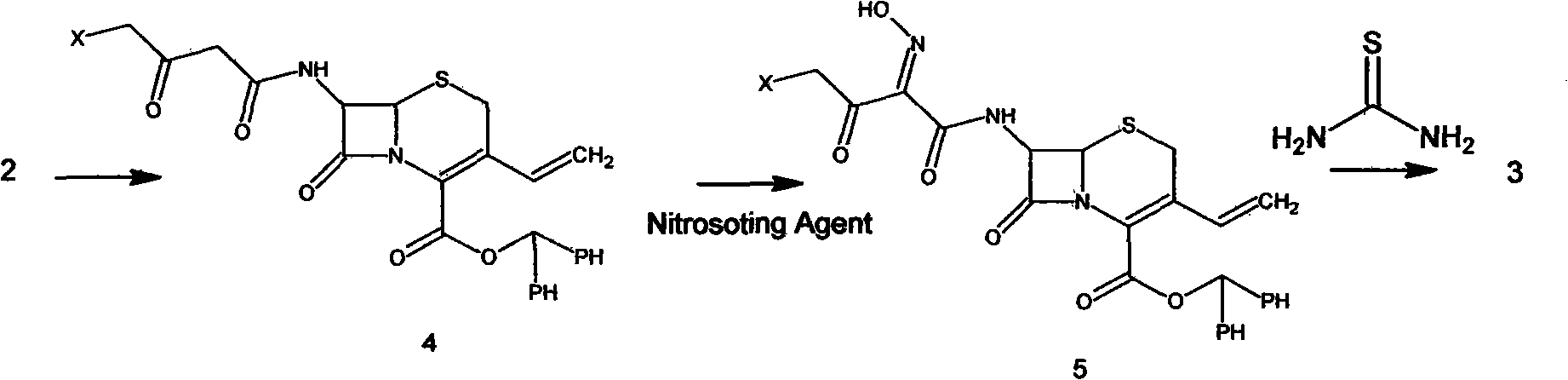
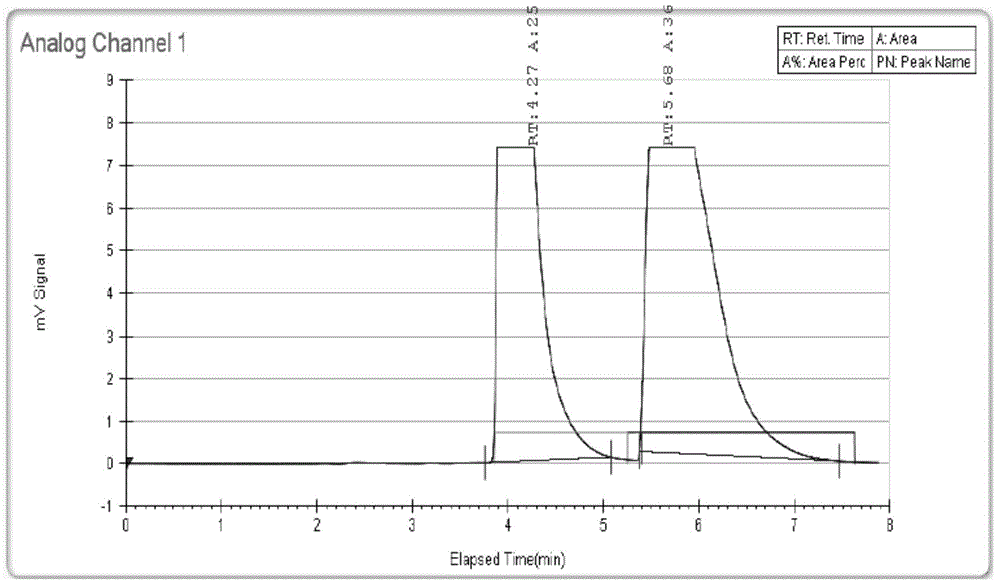
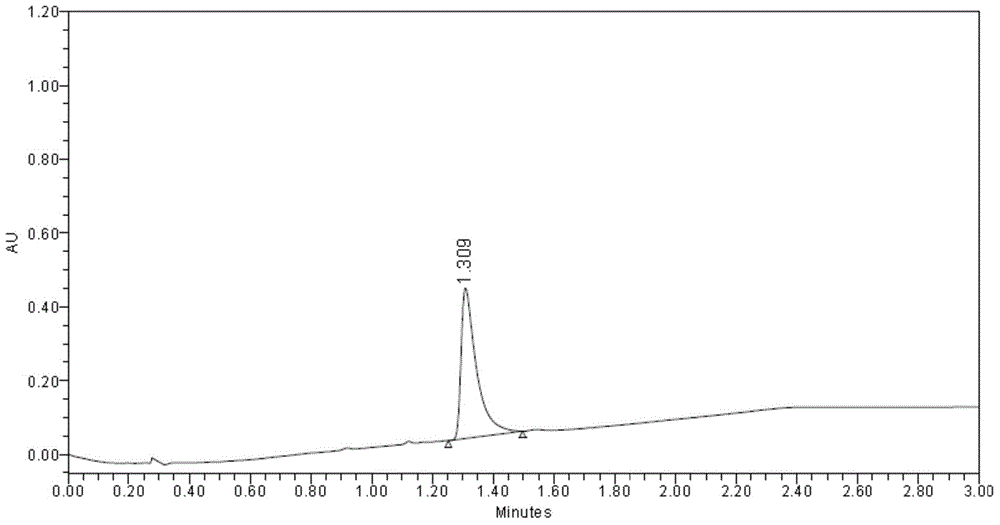
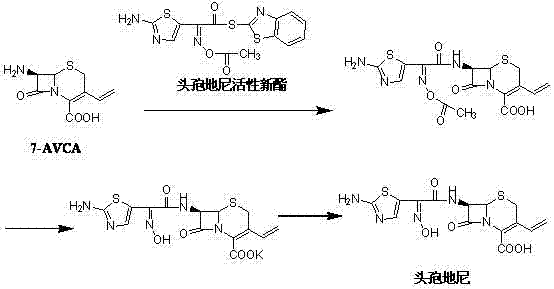
Preparation method of cefdinir
ActiveCN101565427AEasy to recycleReduce pollutionAntibacterial agentsOrganic chemistryOrganic baseCarboxylic acid
The invention relates to a preparation method of cefdinir, comprising the following steps: reacting 7-amino-3-vinyl-8-oxy-5-thia-1- nitric heterocyclic dicyclo[4.2.0]octyl-2-en-2-carboxylic acid with (Z)-2-(2-aminothiazole-4-yl)-2-acetoxy imino thioacetic acid (S-2-benzothiazole)ester in the presence of organic base at low temperature; extracting, adjusting p H value, preparing the intermediate of the cefdinir, removing the ester-group protective group of the intermediate of the cefdinir to obtain the cefdinir. The preparation method uses the low-temperature reaction technique, capable of increasing the reaction yield and reducing the impurities generated by the high temperature reaction. The hydrolysis and crystallization process is very easily controlled. The used alcohols, ketones or esters solvent is easily recovered, thus the production cost and the three-wastes drain are reduced, therefore the pollution to the environment is reduced. The preparation method of cefdinir is suitable for large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
Hydroxylethylidene diphosphonic acid with arsenic content <=3ppm and preparation technique thereof
ActiveCN101386628AReduce arsenic levelsReduce pollutionGroup 5/15 element organic compoundsAcetic acidEtidronic acid
The invention discloses an etidronic acid with the content of arsenic less than or equal to 3ppm and a process for preparing the same. The method is to use phosphorus trichloride, acetic acid and ammonium thioacetamide as raw materials and comprises the following steps of: putting one-third of the acetic acid and the ammonium thioacetamide into a reaction kettle, stirring to control the temperature and the pressure, slowly dropping one-third of the phosphorus trichloride and putting one-third of the ammonium thioacetamide into the reaction kettle, stopping stirring and keeping stand; controlling the temperature and the pressure; dropping the remaining phosphorus trichloride into the reaction kettle; then stirring for 1 hour with the rotational speed of 50 to 60 revolutions per minute, raising the temperature slowly and reflowing; controlling the temperature and the pressure while reflowing; hydrolyzing the esters produced after reflowing under the negative pressure; concentrating the materials, filling air, removing impurities, reducing the temperature, putting the remaining phosphorus trichloride into the reaction kettle, sealing the reaction kettle and keeping stand, and then putting the materials into a mixing tank; regulating and filtering the materials so as to obtain an HEDP product. The product has extremely little arsenic with less than or equal to 3ppm, which not only reduces the environmental pollution, but also expands the application fields.
Owner:HENAN QINGSHUIYUAN TECH
Optically pure thioacetic compound
ActiveCN105985295ALower doseStrong excretion of uric acidOrganic chemistryAntipyreticBromineChemistry
The invention belongs to the field of pharmacy and relates to an optically pure thioacetic compound and application thereof to medicine. The optically pure thioacetic compound comprises levorotatory 2-(5-bromo-4-(4-cyclopropyl-naphthalene-1-base)-4H-1,2,4-triazole-3-based sulfenyl) acetic acid and dextral 2-(5-bromo-4-(4-cyclopropyl-naphthalene-1-base)-4H-1,2,4-triazole-3-based sulfenyl) acetic acid.
Owner:CHINA RESOURCES SAIKE PHARMA
Preparation method of cefdinir
InactiveCN102516261AThe size is easy to controlEasy to wash offOrganic chemistryCarboxylic acidAlkaline hydrolysis
The invention relates to a preparation method of cefdinir. According to the invention, under the effect of triethylamine, 7-amino-3-vinyl-8-oxo-5-thia-1-azabicyclo[4.2.0]octa-2-alkene-2carboxylic acid is subject to a reaction with (Z)-2-(2-aminothiazole-4-group)-2-acetyl oxyimino thioacetic acid(S-2-benzothiazole), such that a cefdinir ester liquid is obtained; the cefdinir ester liquid is extracted; an organic solvent is added to the cefdinir ester liquid; acetyl is removed by alkaline hydrolysis; sylvite of a weak acid is added to the liquid, the pH value is controlled, such that cefdinir sylvite is obtained; the sylvite is dissolved by using water; an organic solvent is added to the solution; the pH value is regulated, such that cefdinir is obtained. With the method, yield and quality are substantially improved; crystal form of the product is stable; and the method is suitable for industrialized productions.
Owner:ZHEJIANG GUOBANG PHARMA
Novel fried wheat essence
InactiveCN101602983ACover up moldy smellGreat tasteEssential-oils/perfumesFood scienceTetramethyl pyrazine2-acetylthiophene
The invention discloses a novel fried wheat essence, comprising 2-acetylthiophene, furanone, 2,3-pentanedione, 3-methyl-2-cyclohexenyl-1-one, 2,3,5,6-tetramethylpyrazine, 5-methyl-6,7-dihydrocyclopentylpyrazine, 2-acetylpyrazine, tricin, furfuryl thioacetate, biradical disulfide and propylene glycol. The invention not only has fried wheat fragrance and but also can be used in the coarse grain beverage after baking, in particular to wheat smell milk beverage. Meanwhile, the invention can better enhance the mouthfeel and cover the bilgy odour and musty taste of legume coarse grain in base materials.
Owner:广州市凯虹香精香料有限公司
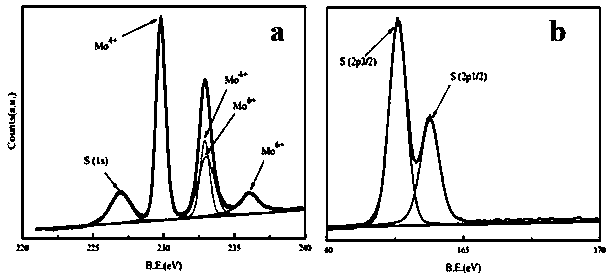
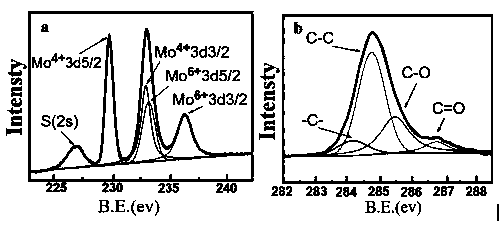
Preparation method and application of copper sulphide/molybdenum sulfide composite
ActiveCN109569665AGood repeatabilityMild reaction conditionsHydrocarbon by hydrogenationCatalystsReaction temperatureOne-pot synthesis
The invention discloses a preparation method and application of a copper sulphide / molybdenum sulfide composite. According to the preparation method, raw materials, namely (NH4)6Mo7O24.4H2O, Cu(CH3COO)2 and 1,3-di(thioacetic acid-S-n-propyl)imidazolium bromide are subjected to one-pot synthesis by a hydrothermal process to obtain the copper sulphide / molybdenum sulfide composite; preparation condition of the composite is evaluated according to the catalytic hydrogenation effect to naphthalene, and reaction temperature and time and proportion of raw materials in preparation of the composite are optimized; particularly, on the optimized reaction conditions that the mass ratio of Mo, Cu to S of (NH4)6Mo7O24.4H2O, Cu(CH3COO)2 and 1,3-di(thioacetic acid-S-n-propyl)imidazolium bromide is 1:1: 2.5,the reaction temperature is 160 DEG C and the reaction time is 8 hours, the catalytic hydrogenation conversion rate of the copper sulphide / molybdenum sulfide composite prepared by the method to naphthalene is up to 55.6%. In the reaction process, 1,3-di(thioacetic acid-S-n-propyl)imidazolium bromide functional ionic liquid can serve as not only a sulfur source but a surfactant, and thus use of raw materials and cost are both reduced.
Owner:DONGGUAN UNIV OF TECH
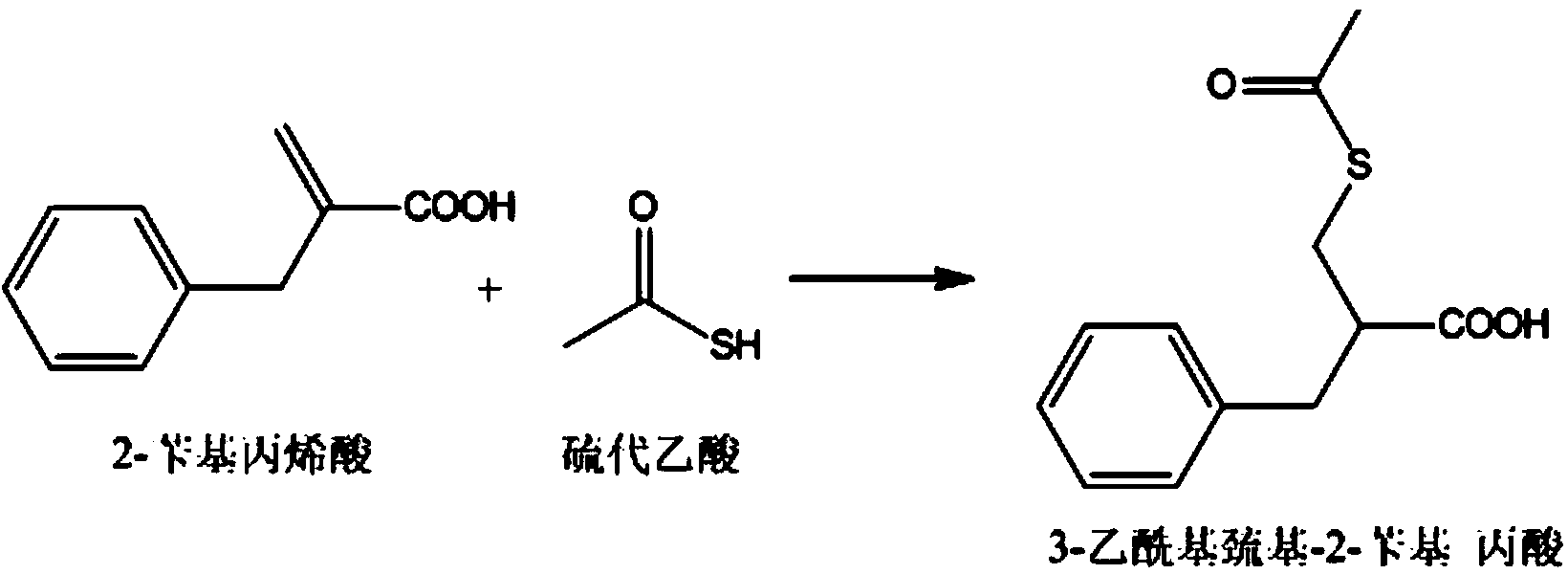
Preparation method of racecadotril
The invention relates to a preparation method of racecadotril. The method comprises the following steps: (1) enabling thioacetic acid to react with 2-benzylacrylic acid to prepare an intermediate 3-acetyl mercapto-2-benzyl propanoic acid; (2) carrying out amidation on the intermediate 3-acetyl thiol-2-glycin benzyl propionic acid and glycine benzyl ester p-toluenesulfonate salt under existence of a catalyst to generate the racecadotril. By adopting the method, the racecadotril with high yield and purity can be obtained, the defect of the original technology is compensated, pollution is reduced, and the cost is reduced. Thioacetic acid is used as a solvent and is also used as a reaction reagent, reaction process is simple and rapid, few reagents are used, and the purity and the yield are greater than or equal to 95%; EDCI or DCC is selected as a condensation reagent; a mixed solvent is used for crystallization, the influence on product yield is small, and impurity removal effect is good; the overall technology adopts a one-step to synthetize, simple, efficient, high in atom economy, more green and environment-friendly, and the racecadotril product which is high in yield, and accords with the EP quality standard can be obtained.
Owner:陕西汉江药业集团股份有限公司
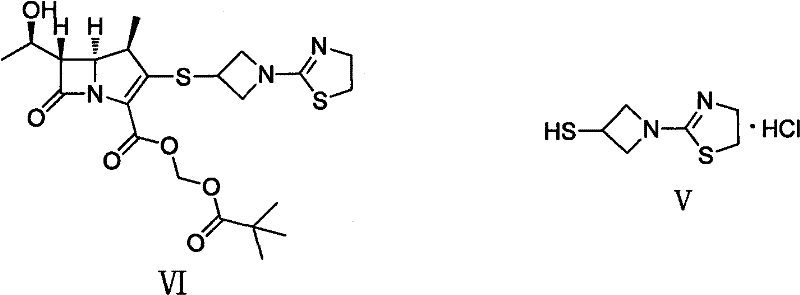
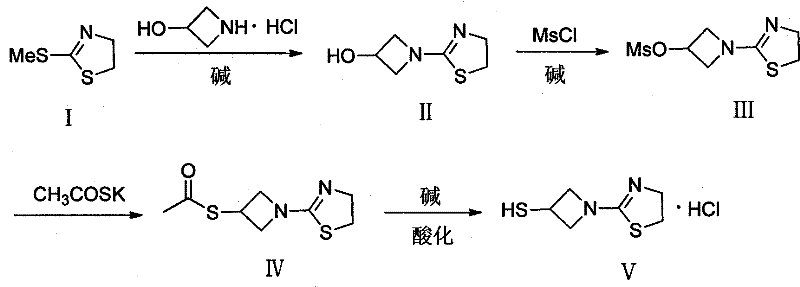
Preparation method of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride
ActiveCN102250080ARaw materials are easy to getSimple process routeOrganic chemistryHydrogenMethane sulfonate
The invention discloses a method for synthesis of 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride. With 2-methylthio-2-thiazoline (I) as the raw material, the method of the invention comprises the steps of: in the presence of alkali, reacting 2-methylthio-2-thiazoline (I) with 3-hydroxyazetidine hydrochloride so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-hydroxyazetidine (II), and reacting (II) with methyl sulfonylchloride in the presence of alkali so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-methane sulfonate group azetidine (III), subjecting (III) to a reaction with potassium thioacetate so as to obtain 1-(4, 5-dihydro-2-thiazolinyl)-3-thioacetic acid ester group azetidine (IV), in the presence of alkali, subjecting (IV) to hydrolysis and then to acidification with dilute hydrochloric acid, thus obtaining the 1-(4, 5-dihydro-2-thiazolinyl)-3-mercaptoazetidine hydrochloride (V). With easily available and inexpensive starting material, the method of the invention simplifies the synthesis route, improves the raw material utilization ratio and the overall yield. An intermediate obtained in the reaction can be subjected to refinement by a recrystallization method or to a next reaction directly, so that the yield is high and the "three wastes" produced during the reaction process are few. In addition, with low cost, the method of the invention is beneficial for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Method for synthesizing dithiothreitol
InactiveCN101503384AAvoid it happening againEasy to separate and purifyThiol preparationEthylene oxideBromine
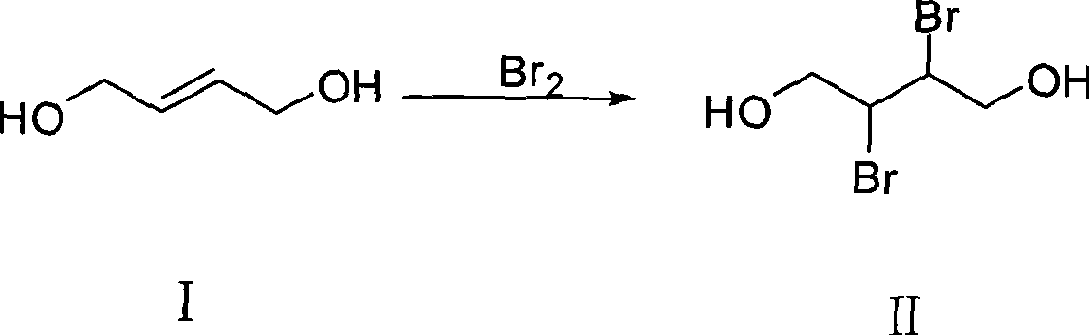
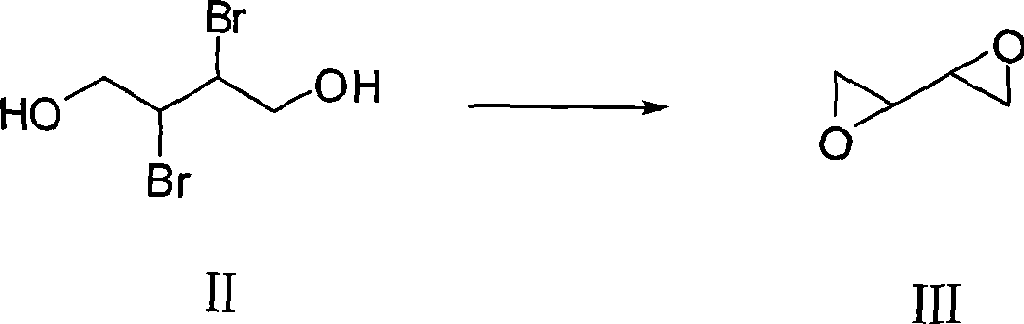
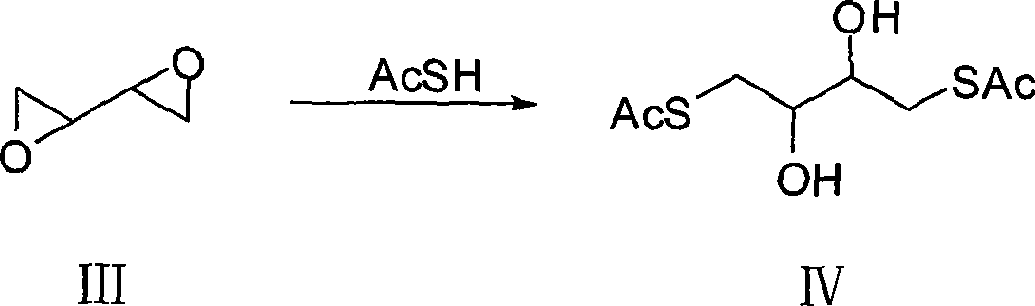
The invention discloses a synthesizing method of dithiothreitol. 1,4-butylene glycol is used as an initiating raw material and undergoes addition reaction with bromine at first to obtain 2,3-dibromo-1,4-butylene glycol; hydrolyzation under the catalyzation of alkali is carried out to obtain dioxirane; addition reaction with thioacetic acid is carried out to obtain dithiothreitol diacetate; and finally hydrolyzation under the catalyzation of alkali is carried out to obtain the dithiothreitol. The synthesizing method avoids the generation of dithiothreitol isomeride impurities, simplifies the product separation and purification, and can improve the yield rate, greatly reduce the production cost and is applicable to industrial production.
Owner:CHONGQING BORNING CHEM & INDAL
Preparation method of cefdinir
ActiveCN101565427BEasy to recycleReduce pollutionAntibacterial agentsOrganic chemistryOrganic baseCarboxylic acid
The invention relates to a preparation method of cefdinir, comprising the following steps: reacting 7-amino-3-vinyl-8-oxy-5-thia-1- nitric heterocyclic dicyclo[4.2.0]octyl-2-en-2-carboxylic acid with (Z)-2-(2-aminothiazole-4-yl)-2-acetoxy imino thioacetic acid (S-2-benzothiazole)ester in the presence of organic base at low temperature; extracting, adjusting pH value, preparing the intermediate ofthe cefdinir, removing the ester-group protective group of the intermediate of the cefdinir to obtain the cefdinir. The preparation method uses the low-temperature reaction technique, capable of increasing the reaction yield and reducing the impurities generated by the high temperature reaction. The hydrolysis and crystallization process is very easily controlled. The used alcohols, ketones or esters solvent is easily recovered, thus the production cost and the three-wastes drain are reduced, therefore the pollution to the environment is reduced. The preparation method of cefdinir is suitable for large-scale production.
Owner:ZHEJIANG ANGLIKANG PHARMA
Preparation method of cefodizime acid
The invention belongs to the technical field of medicament synthesis, and particularly relates to a preparation method of cefodizime acid. The preparation method is characterized by comprising the following steps of: adding 7-aminocephalosporanic acid, 2-Mercapto-4-methyl-1,3-thiazol-5-yl-acetic acid(MMTA) and water, dropwise adding an alkaline solution, adjusting pH, adding an organic solvent, stirring and mixing, and adjusting pH by using glacial acetic acid; leaching, rinsing by the organic solution, and drying; directly adding the dried substance obtained from the former step into a dichloromethane solution after rinsing, adding 2-methoxy imino-2-(2-azyl-4-thiazolyl)-(z)-thioacetic acid phenylhydrazine thiazole ester, and dropwise adding a 7-aminocephalosporanic acid basic catalyst for reaction; and extracting with water, and adjusting pH with glacial acetic acid to obtain cefodizime acid. After the special alkaline solution is used, the reaction yield of 7-aminocephalosporanic acid and MMTA can be improved. After the amide basic catalysts are used, the product quality of synthesized cefodizime acid can be guaranteed.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD +1
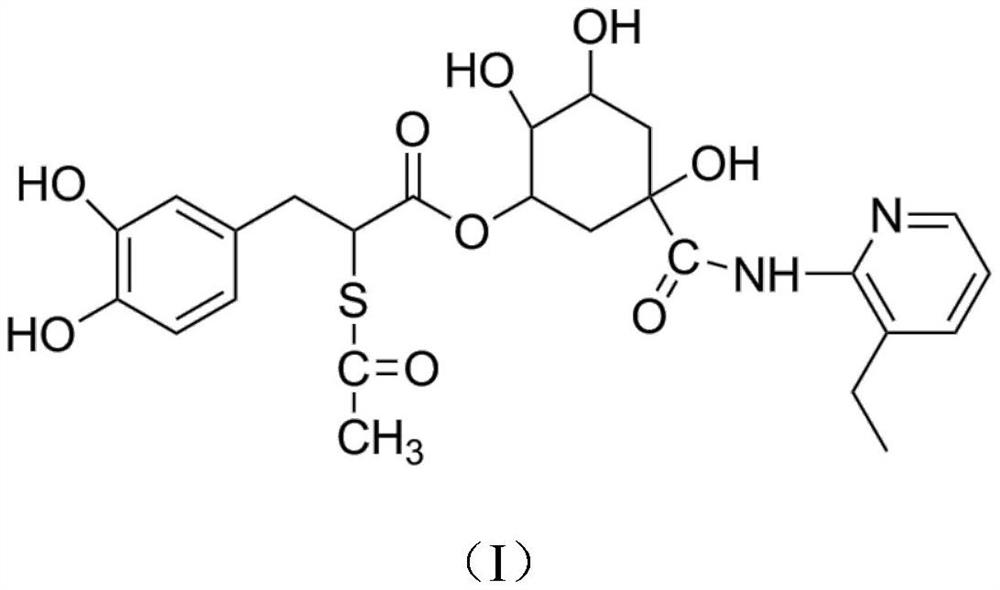
Chlorogenic acid derivative capable of clearing away heat and toxic materials and preparation method thereof
InactiveCN112851576AEnhanced inhibitory effectStrong inhibitory activityAntibacterial agentsAntimycoticsChlorogenic acidPtru catalyst
The invention discloses a chlorogenic acid derivative capable of clearing away heat and toxic materials and a preparation method thereof, and belongs to the technical field of synthesis and preparation of chemical drugs. The preparation method of the derivative comprises the following steps: under the action of a catalyst, carrying out esterification on chlorogenic acid and methanol, then carrying out ester ammonolysis with 2-amino-3-ethylpyridine, carrying out double-bond addition with thioacetic acid, and purifying a crude product through a silica gel column to obtain the chlorogenic acid derivative. According to the invention, the structure of chlorogenic acid is modified, a compound with a more novel structure and stronger activity is found, and the compound has remarkable effects of clearing away heat and toxic materials, resisting bacteria and diminishing inflammation and the like; and the chlorogenic acid derivative prepared by the method disclosed by the invention is high in yield, simple in process, mild in reaction condition and good in yield and selectivity, and has economic characteristics of an industrial application environment.
Owner:谢天龙
Method for preparing carbon-doped molybdenum sulfide/graphene oxide composite material
ActiveCN109622057ALow costThe preparation method is simple and controllableOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon by hydrogenationOxide compositeOne-pot synthesis
The invention discloses a method for preparing a carbon-doped molybdenum sulfide / graphene oxide composite material. The method takes (NH4) 6Mo7O24 4H2O, graphene oxide, 1,3-di(thioacetic acid-S-n-propyl) imidazole bromide and ethyl alcohol as raw materials, and comprises the steps of a, preparation of a mixed solution, b, heating, c, cooling, d, filtering, and e, drying and storing, wherein one-pot synthesis is carried out through a hydrothermal method to obtain the carbon-doped molybdenum sulfide / graphene oxide composite material. The method for preparing the carbon-doped molybdenum sulfide / graphene oxide composite material has the advantages that through the synergistic effect of a 1,3-di(thioacetic acid-S-n-propyl) imidazole bromide dimercapto functionalized ionic liquid, the graphene oxide and the ethyl alcohol, the hydrogenation conversion rate of the composite material for naphthalene reaches up to 45.8%, the preparation method is simple and controllable, the cost of the raw materials is lower, and the composite material is green and has no pollution.
Owner:DONGGUAN UNIV OF TECH
Use Of Thioacetic Acid Derivatives In The Sulfurization Of Oligonucleotides With Phenylacetyl Disulfide
A method and compositions for sulfurizing at least one phosphite or thiophosphite linkage in an oligonucleotide. The methods employ a phenylacetyl disulfide reagent (known as PADS), phenylthioacetic acid (PTAA) in the presence or absence or N-alkyl imidazole in industrially preferred solvents or solvents that are derived from renewable resources. The use of PTAA eliminates the need to “age” the PADS solution prior to its use in sulfurization reactions.
Owner:AGILENT TECH INC
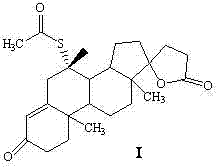
Synthetic method of spironolactone
ActiveCN102321139BReduce pollutionPromote sustainable developmentSteroidsChemical synthesisAcetic acid
A synthetic method of spironolactone belongs to the technical field of chemical synthesis. The method comprises the following steps: heating and refluxing for 3-5 hours to perform an addition reaction with canrenone and potassium thioacetate as raw materials and ethanol as a solvent in the presence of an acidic catalyst, after the reaction, cooling to -10 DEG C, performing heat preservation for 1.5-2.5 hours, filtering, treating the filter cake to obtain spironolactone, wherein the feeding molar ratio of canrenone, potassium thioacetate, and the acidic catalyst is 1:2.1:2.1. The invention adopts thiacetate instead of thioacetic acid, which effectively solves the environmental protection pressure caused by thioacetic acid, and reduces environment pollution; the method has a simple process, is easy to operate, and has high economic and environmental protection benefit; the invention mainly solves the technical problem of existing synthetic methods that the special smell of the raw material of thioacetic acid causes high environmental protection pressure for enterprises.
Owner:ZHEJIANG LANGHUA PHARMA
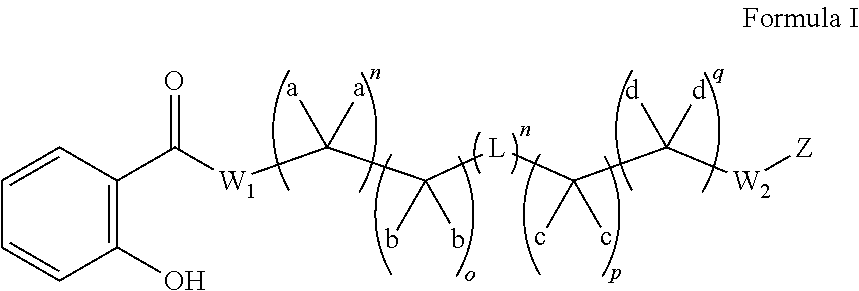
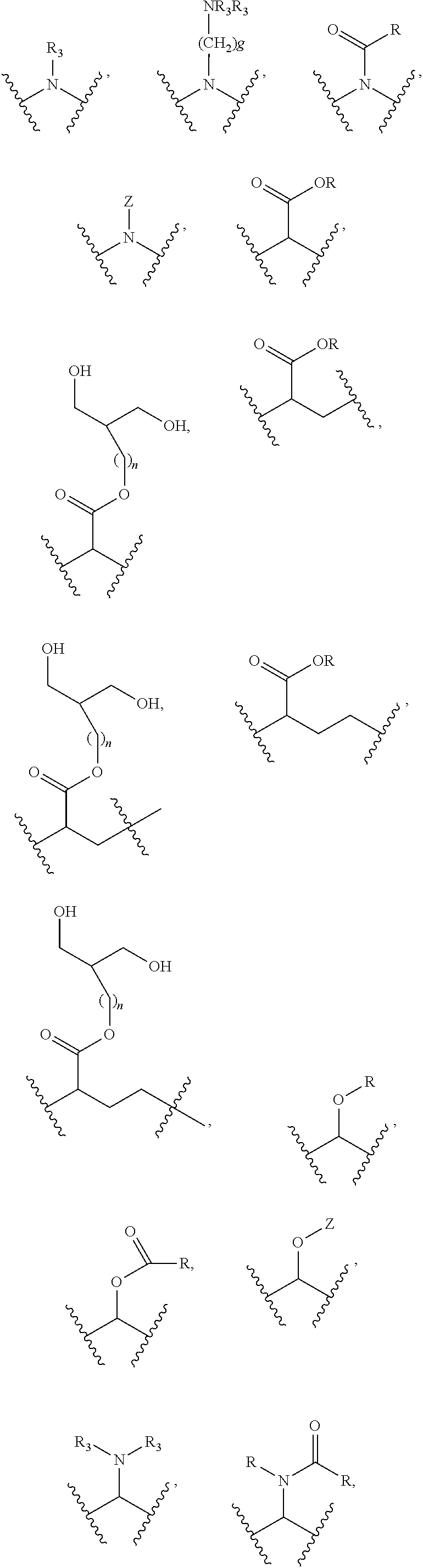
Substituted thioacetic acid salicylate derivatives and their uses
InactiveUS20110082120A1Convenient treatmentElevated cholesterolBiocideSalicyclic acid active ingredientsMedicinal chemistryEster derivatives
The invention relates to substituted thioacetic acid salicylate derivatives; compositions comprising an effective amount of a substituted thioacetic acid salicylate derivative; and methods for treating or preventing an metabolic disease comprising the administration of an effective amount of a substituted thioacetic acid salicylate derivative.
Owner:CATABASIS PHARMA
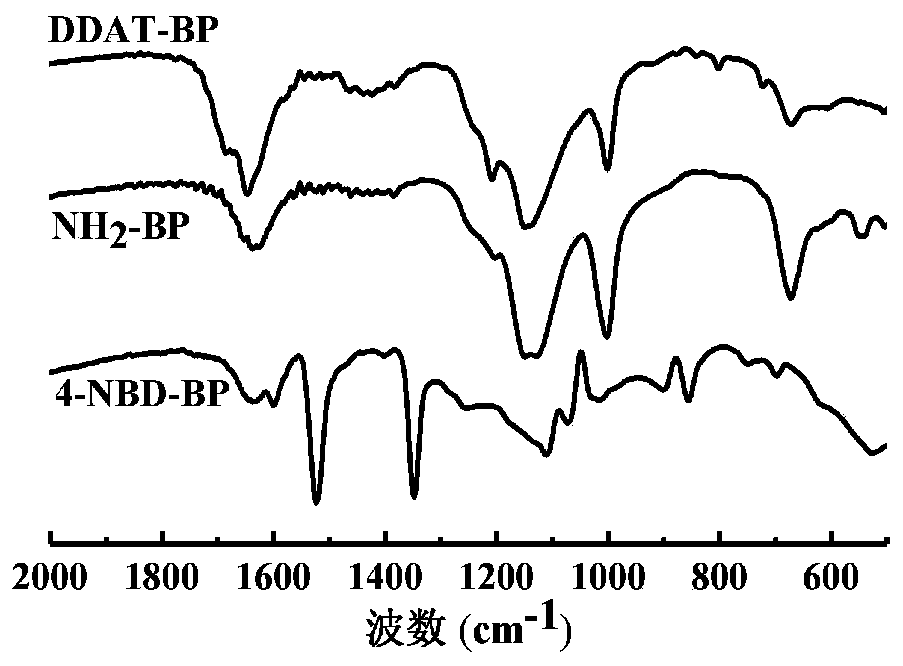
Novel reversible additive-fragment transfer (RAFT) reagent based on black phosphorus and preparation method and application thereof
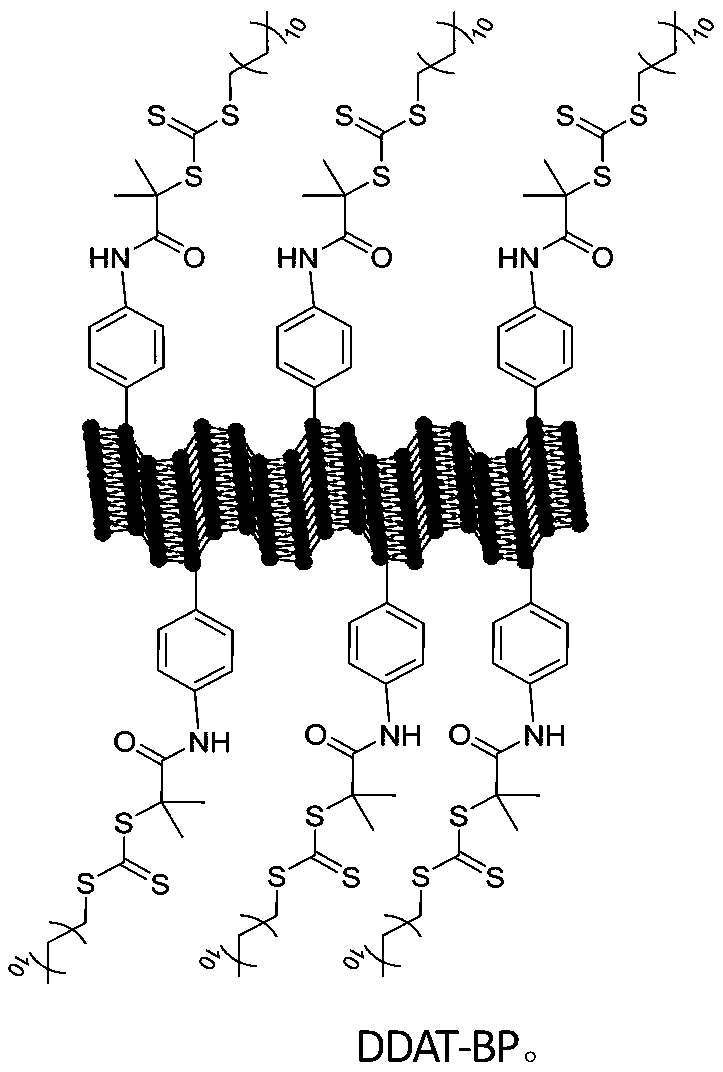
The invention belongs to a method for preparing a novel reversible additive-fragment transfer (RAFT) reagent based on black phosphorus, and the method includes the steps that a RAFT reagent containingcarboxyl groups in molecules is grafted onto BP nano sheets by covalent modification, and the RAFT reagent based on black phosphorus is obtained. The preparation method is applicable to various RAFTreagents with carboxyl groups in the molecules and can be effectively applied to a series of RAFT polymerized monomers, a platform for the modification of black phosphorus nano sheets is provided; meanwhile the polymer modified black phosphorus nano sheet material by the material in the preparation method has outstanding solubility and charge transfer properties compared with pure black phosphorusnano sheets, so that the polymer modified black phosphorus nano sheet material has a wide application prospect in photoelectric devices. The preparation method is applicable to the RAFT reagents containing carboxyl groups in molecules such as s-1-dodecyl-s'-(a,a'-dimethyl-a''-acetoxy) tristhiocarbonate (DDAT), 4-cyano-4-[(dodecyl thiocarbonyl) thioalkyl]valeric acid, 4-cyano-4-(phenylthioformyl)valeric acid, S-(thiobenzoyl) thioacetic acid.
Owner:EAST CHINA UNIV OF SCI & TECH
Surface modification of CVD polymer films
ActiveUS7501154B2Liquid surface applicatorsSemiconductor/solid-state device manufacturingTetramethylammonium hydroxideXylylene
The present invention relates to a method for forming a conformal coating having a reactive surface. In the method, an ultrathin layer composed of a polymer having repeating units derived from unsubstituted p-xylylene, substituted p-xylylene, phenylene vinylene, phenylene ethynylene, 1,4-methylene naphthalene, 2,6-methylene naphthalene, 1,4-vinylene naphthalene, 2,6-vinylene naphthalene, 1,4-ethynylene naphthalene, 2,6-ethynylene naphthalene, combinations thereof, precursors therefor or combinations of precursors therefor, is deposited on a substrate by a thermal CVD process. The ultrathin layer is optionally exposed to a source of oxygen and then exposed to a reagent selected from ammonium hydroxide, tetramethylammonium hydroxide, ammonium sulfide, dimethyl sulfide, thioacetic acid, sodium hydrosulfide, sodium sulfide, hydrazine, acetamide and combinations thereof. The surface may be modified readily after the treatment.
Owner:RENESSELAER POLYTECHNIC INST
Preparation process for high-purity low-arsenic hydroxyethylidene diphosphonic acid
ActiveCN103509051ARealize continuous online productionMild process control conditionsGroup 5/15 element organic compoundsThio-Ethylic acid
The invention relates to a preparation process for high-purity low-arsenic hydroxyethylidene diphosphonic acid. The preparation process comprises the following steps: with phosphorus trichloride, acetic acid and ammonium thioacetate as raw materials, controlling process conditions at first, reacting acetic acid with phosphorus trichloride in a reaction vessel to produce hydroxyethylidene diphosphonic acid and adding ammonium thioacetate in the process of the reaction; and then subjecting an obtained material to concentration and air flushing to remove impurities, adding ammonium thioacetate and carrying out arsenic removal and centrifugation so as to obtain the high-purity low-arsenic hydroxyethylidene diphosphonic acid. According to the invention, scientific technology is employed for product purification, no other substance like a crystal nucleus and a crystallization promoter is added, so no new impurity is introduced. Hydroxyethylidene diphosphonic acid in the high-purity low-arsenic hydroxyethylidene diphosphonic acid prepared in the invention is more than 95%, and arsenic content in the high-purity low-arsenic hydroxyethylidene diphosphonic acid is less than 2 ppm; the preparation process overcomes problems in packaging, transporting and on-site application of liquid hydroxyethylidene diphosphonic acid and broadens the application fields of the high-purity low-arsenic hydroxyethylidene diphosphonic acid.
Owner:HENAN QINGSHUIYUAN TECH
Process for the preparation of 2-[(diphenylmethyl) thio] acetamide
InactiveUS20060128812A1High yieldReduce usageOrganic active ingredientsBiocideOrganic acidAcetic acid
Process for the preparation of 2-[(diphenylmethyl)thioacetamide, an intermediate for the preparation of Modafinil which is a CNS stimulant and used for the treatment of narcolepsia. The process comprises reacting 2-[(diphenylmethyl)thio]acetic acid with alcohols, in presence of catalytic amount of inorganic acid or organic acid at reflux temperature of alcohol to obtain corresponding ester which is reacted with ammonia to give 2-[(diphenylmethyl)thio]acetamide. If desired 2-[(diphenylmethyl)thioacetamide thus produced is reacted with hydrogen peroxide to produce Modafinil.
Owner:ALEMBIC LTD
Method for preparing substituted homotaurine from alpha, beta-unsaturated amide
ActiveCN102659643AEasy to operateSuitable for large-scale industrial productionOrganic chemistryOrganic compound preparationHomotaurineThio-
The invention provides a method for preparing substituted homotaurine from an alpha, beta-unsaturated amide. The method is characterized in that thioacetic acid and the alpha, beta-unsaturated amide undergo an addition reaction to produce corresponding 3-acetylthioacetamide and through reduction and oxidation, the substituted homotaurine is obtained. The method adopts simple and easily available raw materials, is convenient for operation, avoids a complicated desalting purification process, and is especially suitable for large-scale industrial production. The homotaurine can be used as a nutriment, a drug, an enzyme inhibitor, an antiseptic, a surfactant, a plant-growth regulator and a raw material of sulfonopeptides.
Owner:BEIJING UNIV OF CHEM TECH
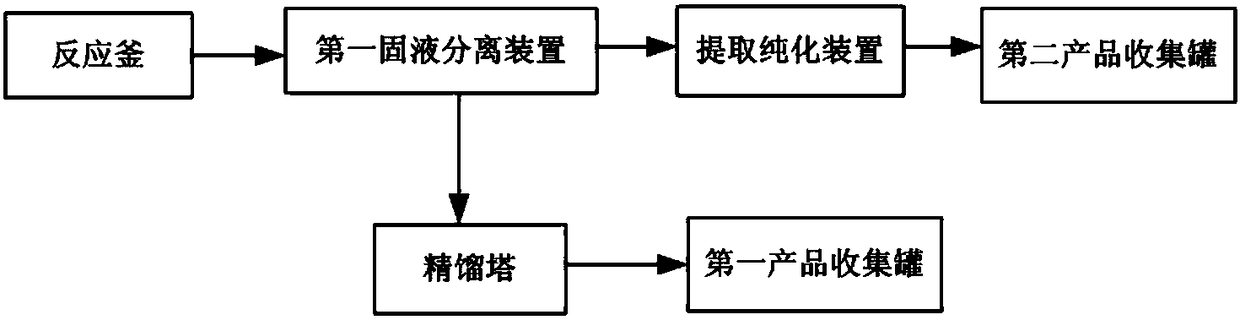
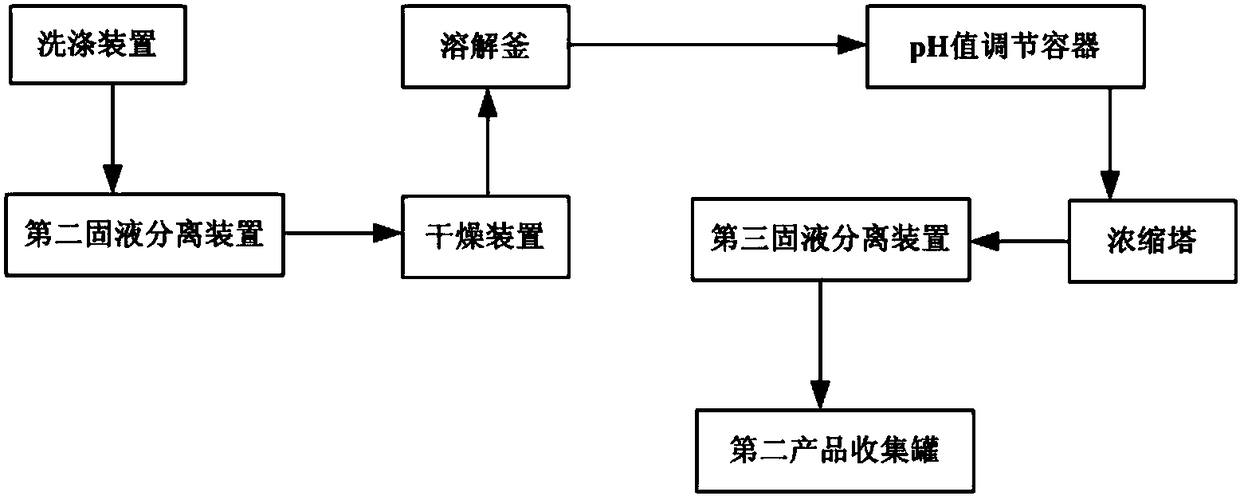
Preparation method and production device of thioacetic acid
PendingCN108314637AHigh purityHigh yieldOrganic chemistryPhosphorus compoundsAcetic acidPhosphorus pentasulfide
The invention discloses a preparation method and a production device of thioacetic acid. The thioacetic acid is obtained through carrying out low temperature reaction by adopting acetic acid and phosphorus pentasulfide under the action of a specific catalyst, carrying out solid-liquid separation, and carrying out distillation separation on a liquid phase. A solid-phase substance obtained through solid-liquid separation for the first time is further extracted and purified, so that a byproduct potassium phosphate is obtained. Low-price and easy-to-get raw materials and the catalyst are adopted and can be reacted to synthesize to obtain the thioacetic acid at lower temperature and pressure intensity and even at normal pressure and temperature, solid-liquid separation is carried out to obtainthe liquid phase, and the liquid phase is subjected to distillation separation so as to obtain the thioacetic acid with high purity and high yield. Through further extracting and purifying the solid-phase substance obtained through solid-liquid separation for the first time, the high-value-added byproduct potassium phosphate with high purity and high yield is obtained from the waste liquid, so that the preparation method is friendly to the environment and has higher economical efficiency.
Owner:兖矿水煤浆气化及煤化工国家工程研究中心有限公司 +1
Catalyst-free technique for synthesizing phenothiazine drug intermediate
ActiveCN105669590ASimple and fast operationMild reaction conditionsOrganic chemistryRegioselectivityThio-
The invention provides a catalyst-free excellent-regioselectivity weak-alkali-prommoted technique for synthesizing a phenothiazine intermediate. The method comprises the following step: in an inert atmosphere, 2-acetaminophenyl S-thioacetate and o-dihalogenated aromatic hydrocarbons directly react in the presence of a weak alkali to obtain the phenothiazine drug intermediate. The technique is green and environment-friendly, has higher reaction yield of the intermediate for most phenothiazines, and has industrialized application prospects.
Owner:BEIJING FANBO BIOCHEM
Synthetic method of spironolactone
ActiveCN102321139AReduce pollutionPromote sustainable developmentSteroidsChemical synthesisAcetic acid
A synthetic method of spironolactone belongs to the technical field of chemical synthesis. The method comprises the following steps: heating and refluxing for 3-5 hours to perform an addition reaction with canrenone and potassium thioacetate as raw materials and ethanol as a solvent in the presence of an acidic catalyst, after the reaction, cooling to -10 DEG C, performing heat preservation for 1.5-2.5 hours, filtering, treating the filter cake to obtain spironolactone, wherein the feeding molar ratio of canrenone, potassium thioacetate, and the acidic catalyst is 1:2.1:2.1. The invention adopts thiacetate instead of thioacetic acid, which effectively solves the environmental protection pressure caused by thioacetic acid, and reduces environment pollution; the method has a simple process, is easy to operate, and has high economic and environmental protection benefit; the invention mainly solves the technical problem of existing synthetic methods that the special smell of the raw material of thioacetic acid causes high environmental protection pressure for enterprises.
Owner:ZHEJIANG LANGHUA PHARMA
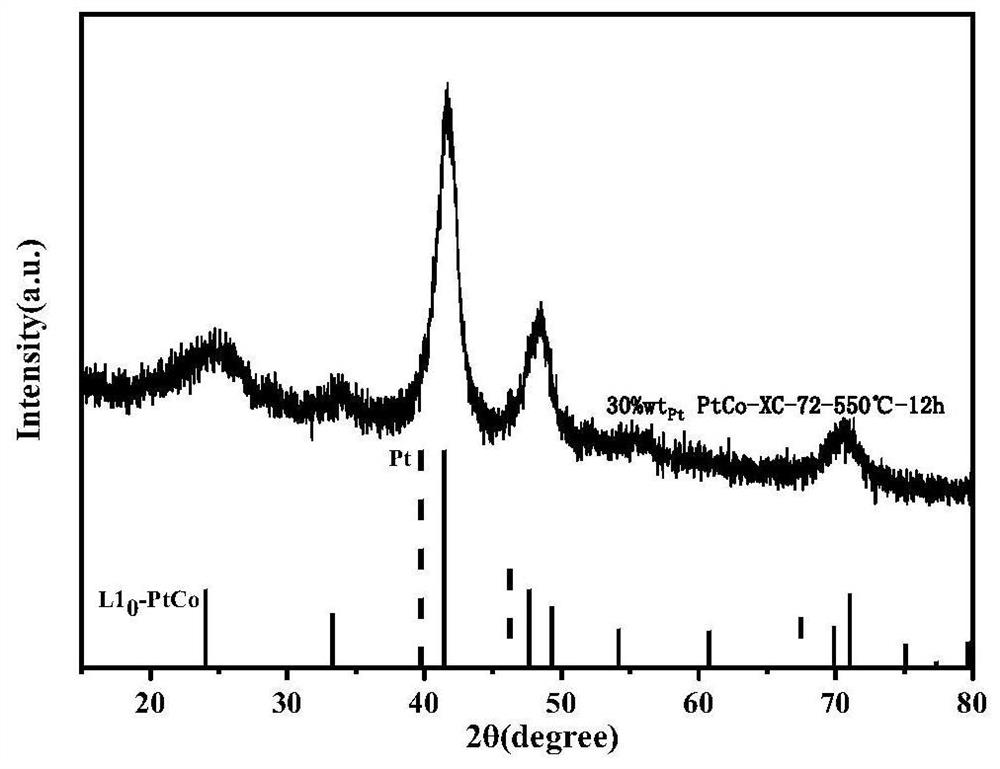
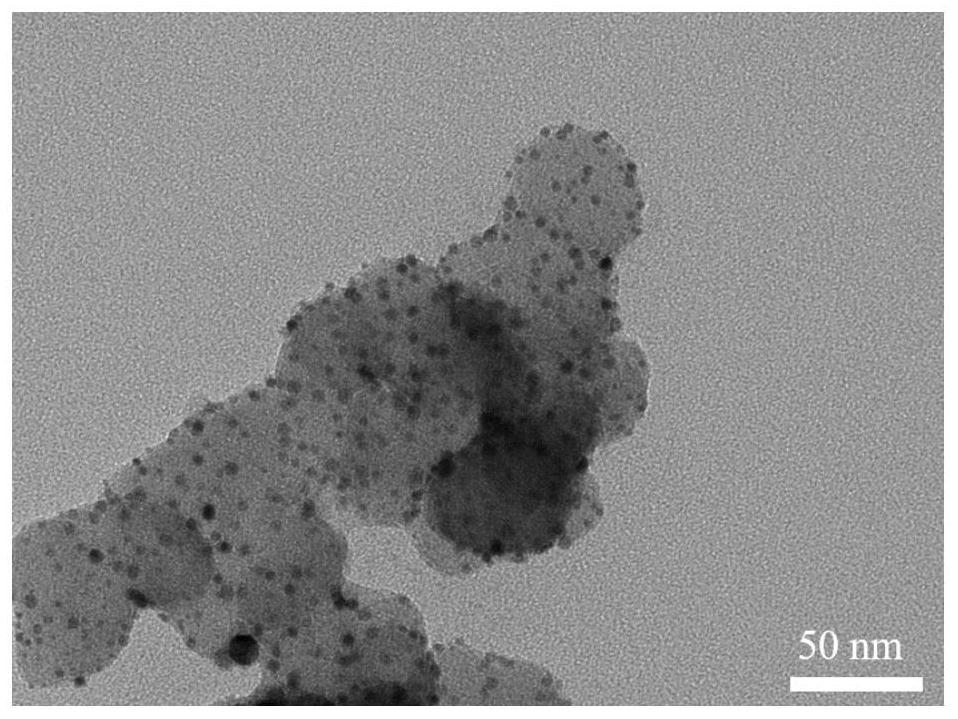
Intermetallic compound catalyst and method for preparing intermetallic compound catalyst by using bimetallic complex
ActiveCN113398951AHigh degree of atomic alloyingComposition is easy to controlCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsSodium bicarbonatePlatinum
The invention provides a method for preparing an intermetallic compound catalyst by using a bimetallic complex. The method comprises the following steps: S1) mixing thioacetic acid, sodium bicarbonate, potassium chloroplatinite and a transition metal salt in an aqueous solution to obtain bimetallic complex molecules separated out in a precipitate form; s2) carrying out moisture removal treatment on the bimetallic complex molecules; s3) mixing the dewatered bimetallic complex molecules with carbon black in a mixed solvent of tert-butyl alcohol and water, and freeze-drying to obtain a mixture of the bimetallic complex molecules and the carbon black; and S4) calcining the mixture in a reducing atmosphere to obtain the intermetallic compound catalyst. The intermetallic compound catalyst prepared by using a freeze drying method can form high-loading platinum load at a low annealing temperature, the atomic ratio of platinum to transition metal is clear 1: 1, the composition is controllable, the size distribution is uniform, and the atomic alloying degree is high. The method is simple to operate, has universality and is suitable for industrial production.
Owner:UNIV OF SCI & TECH OF CHINA
Method of manufacturing thioacetic acid
InactiveCN101108818AHigh product contentAvoid pollutionOrganic chemistryAcetic anhydrideDistillation
The invention discloses a preparation method of the thioacetic acid. The method is that the sodium sulphide produced by the carbon and the mirabilite is adopted to react with the hydrochloric acid to prepare the hydrogen sulfide, and then under the good air-tightness, the hydrogen sulphide gas processed by the dryer reacts with the acetic anhydride to gain the nearly colorless thioacetic acid with content not less than 95 per cent, boiling range of 88 DEG C. to 91DEG C., heavy metals less than 0.0002 per cent, melting point less than minus 17 DEG C. and flash point less than 1 DEG C. through reduced pressure distillation and rectification. The preparation method has convenient operation, which avoids the pollution on the external environment during production, greatly increases the purification of the product content and the product quality and lowers the production cost.
Owner:WEIFANG HAISHUN CHEM
Preparation method of 3,7-dimethyl-3-acetylthio-6-octenol
InactiveCN108822012ALow costReduce manufacturing costOrganic chemistryRoom temperatureSodium borohydride
The invention relates to a preparation method of 3,7-dimethyl-3-acetylthio-6-octenol. The preparation method comprises the following steps: (1) stirring 3,7-dimethyl-3-acetylthio-6-octenal and sodiumborohydride in a mole ratio being 4:1-3:1 in an aqueous solvent at room temperature for reaction for 2-5 hours, and performing washing with an acid aqueous solution until pH is 7; (2) extracting a reaction solution washed to be neutral in the step (1) with an extracting agent, and drying and concentrating the extracted organic phase to obtain a crude product; (3) purifying the crude product obtained in step (2) to obtain a 3,7-dimethyl-3-acetylthio-6-octenol pure product. Compared with the prior art, the preparation method has the advantages of easily available raw materials, simple preparation process and low cost, and is convenient to operate, environmentally friendly and suitable for industrial production, and the problems of environmental unfriendliness, long reaction route and low yield which are caused by adoption of pyridine and thioacetic acid of traditional methods are solved.
Owner:SHANGHAI INST OF TECH
Fly ash cured heavy metal chelating agent and preparation process thereof
InactiveCN110302491AEasy to makeReduce manufacturing costChemical protectionHeavy metal chelationCarboxylic acid
The invention discloses a fly ash cured heavy metal chelating agent and a preparation process thereof and belongs to the field of chemical preparations. The fly ash cured heavy metal chelating agent is prepared from the following raw materials in parts by weight: 20-40 parts of a polycarboxylic acid ammonium salt, 20-30 parts of dimercaptoethylamine hydrochloride, 15-25 parts of amino methane acetate, 10-20 parts of sulfo-acetate, 30-50 parts of water, 5-10 parts of a surfactant, wherein the surfactant is prepared from the following raw materials in parts by weight: 5-30 parts of isopropyl alcohol amine, 10-40 parts of citric acid, 2-6 parts of a cosolvent and 5-20 parts of a dispersant. The chelating agent disclosed by the invention is not only simple to prepare and low in preparation cost, but also high in chelating efficiency, rich in chelating type and high in market application value.
Owner:安徽安江环保科技有限公司
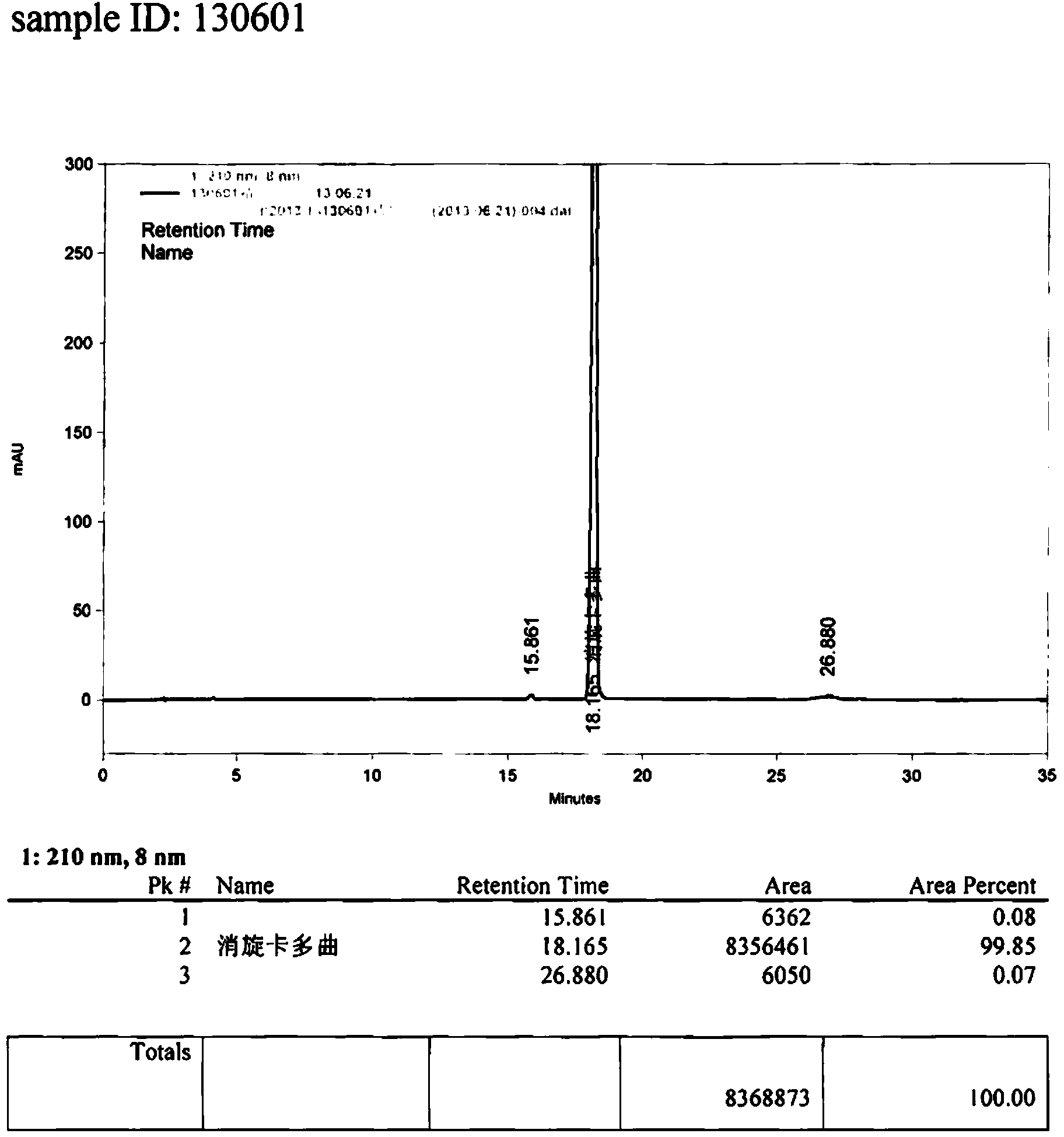
Preparation method of thioacetic acid potassium
This invention relates to a preparation method of thioacetic acid kalium. In existing method, hydrogen sulfide does not has plenary reaction, so utilization ratio is low, result in hydrogen sulfide dosage is large, cost high and three waste are too many, especially in exhaust gas, has serious pollution to environment. This invention uses ketene dimer and sodium polysulfide to inlet hydrogen sulfide gas to absolute alcohol solvent, add antioxidant, after completeness thio reaction, rectify to gain thioacetic acid and absolute alcohol solvent; add thioacetic acid or its hybrid with absolute alcohol solvent by distribution droplets to absolute alcohol solvent of potassium hydroxide, whipping, decompress and reclaim absolute alcohol solvent, and the precipitable white crystal is thioacetic acid kalium.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


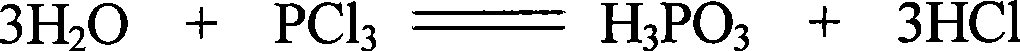
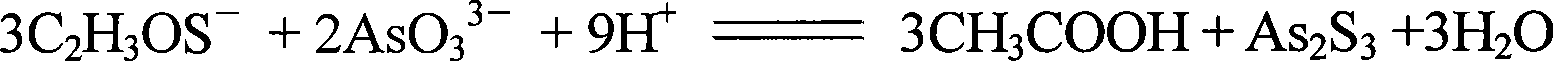
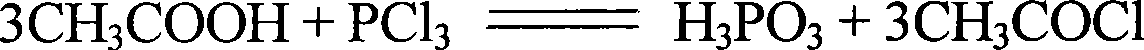
















![Process for the preparation of 2-[(diphenylmethyl) thio] acetamide Process for the preparation of 2-[(diphenylmethyl) thio] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c73e78db-babd-46cf-8b1f-cdcd99c6d2c8/US20060128812A1-20060615-C00001.png)
![Process for the preparation of 2-[(diphenylmethyl) thio] acetamide Process for the preparation of 2-[(diphenylmethyl) thio] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c73e78db-babd-46cf-8b1f-cdcd99c6d2c8/US20060128812A1-20060615-C00002.png)
![Process for the preparation of 2-[(diphenylmethyl) thio] acetamide Process for the preparation of 2-[(diphenylmethyl) thio] acetamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c73e78db-babd-46cf-8b1f-cdcd99c6d2c8/US20060128812A1-20060615-C00003.png)