Method for synthesizing dithiothreitol
A technology of dithiothreitol and a synthesis method, applied in the field of compound synthesis, can solve the problems of difficult separation and purification of DTT, unsuitable for industrialized production, high production cost, etc., and achieves the avoidance of isomer impurities and the simplicity of separation and purification. effect of improving productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The synthetic method of the DTT of the present embodiment, calculates raw material consumption according to product theoretical production amount 277.6g, comprises the following steps:
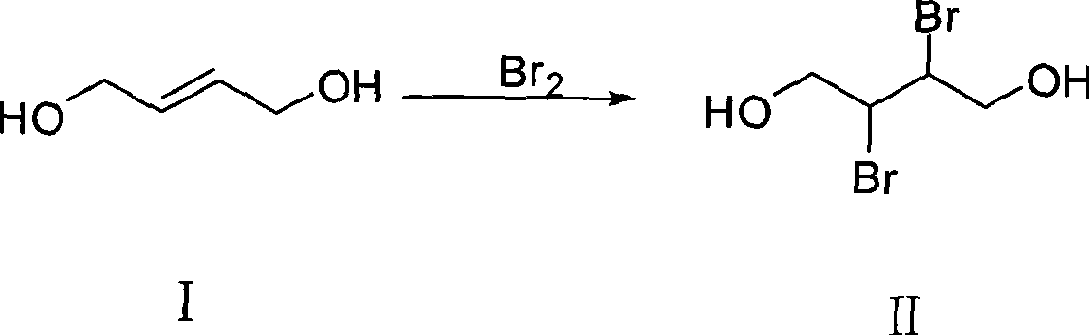
[0027] a. Add 162.5g (1.80mol) of 1,4-butenediol into the reaction kettle, slowly add 316.8g (1.98mol) of liquid bromine dropwise under stirring condition, and stir the reaction at a temperature of 25°C after the dropwise addition After 16 hours, let it stand for stratification, and take the supernatant to obtain 2,3-dibromo-1,4-butanediol, store it in a storage tank with a temperature lower than 30°C, and store it for later use;
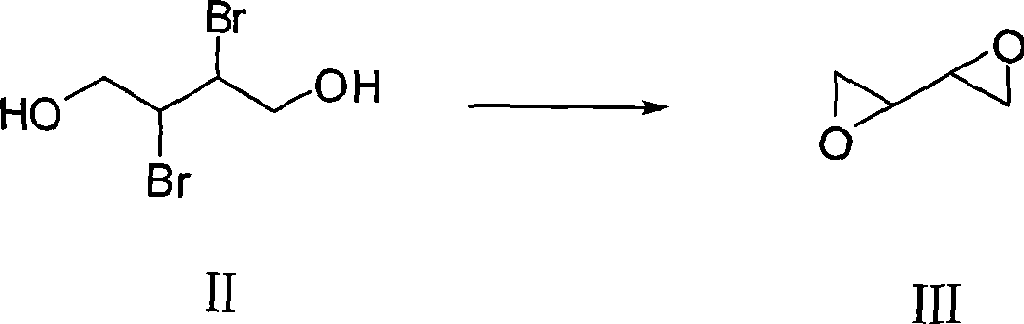
[0028] b. Add 651.2 g (4.88 mol) of 30% sodium hydroxide solution to the supernatant obtained in step a, and stir and react for 16 hours at a temperature lower than 20° C. to obtain dioxirane alkyl;
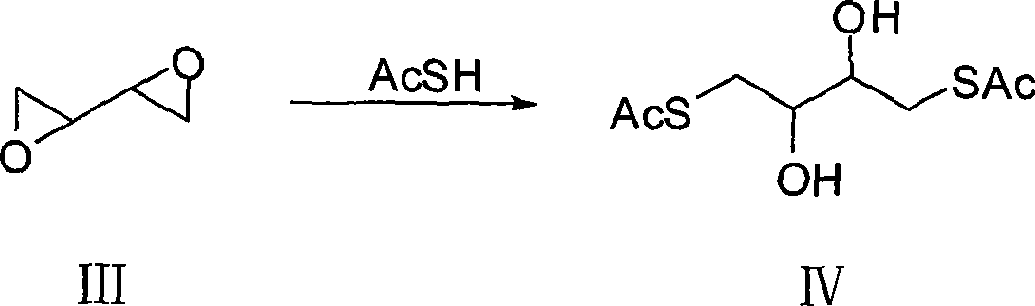
[0029] c. In the reaction solution obtained in step b, add 155.67 g (2.045 mol) of thioacetic acid, stir and react for 20 hours, distill under reduced pressure...
Embodiment 2
[0032] The synthetic method of the DTT of the present embodiment, calculates raw material consumption according to product theoretical production amount 277.6g, comprises the following steps:
[0033] a. Add 162.5g (1.80mol) of 1,4-butenediol into the reaction kettle, slowly add 331.2g (2.07mol) of liquid bromine dropwise under stirring condition, and stir the reaction at a temperature of 27°C after the dropwise addition After 16 hours, let it stand for stratification, and take the supernatant to obtain 2,3-dibromo-1,4-butanediol, store it in a storage tank with a temperature lower than 30°C, and store it for later use;
[0034] b. In the supernatant obtained in step a, add 712.2 g (5.70 mol) of sodium hydroxide solution with a mass percentage concentration of 32%, and stir and react for 16 hours at a temperature lower than 20 ° C to obtain dioxirane alkyl;
[0035] c. In the reaction solution obtained in step b, add 159.38 g (2.094 mol) of thioacetic acid, stir and react for...
Embodiment 3
[0038] The synthetic method of the DTT of the present embodiment, calculates raw material consumption according to product theoretical production amount 277.6g, comprises the following steps:
[0039] a. Add 162.5g (1.80mol) of 1,4-butenediol into the reaction kettle, slowly add 345.6g (2.16mol) of liquid bromine dropwise under stirring conditions, and stir the reaction at a temperature of 30°C after the dropwise addition After 16 hours, let it stand for stratification, and take the supernatant to obtain 2,3-dibromo-1,4-butanediol, store it in a storage tank with a temperature lower than 30°C, and store it for later use;
[0040] b. In the supernatant obtained in step a, add 755.0 g (6.512 mol) of sodium hydroxide solution with a mass percent concentration of 34.5%, and stir and react for 16 hours at a temperature lower than 20°C to obtain dioxirane alkyl;
[0041] c. In the reaction solution obtained in step b, add 163.01 g (2.143 mol) of thioacetic acid, stir and react for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com