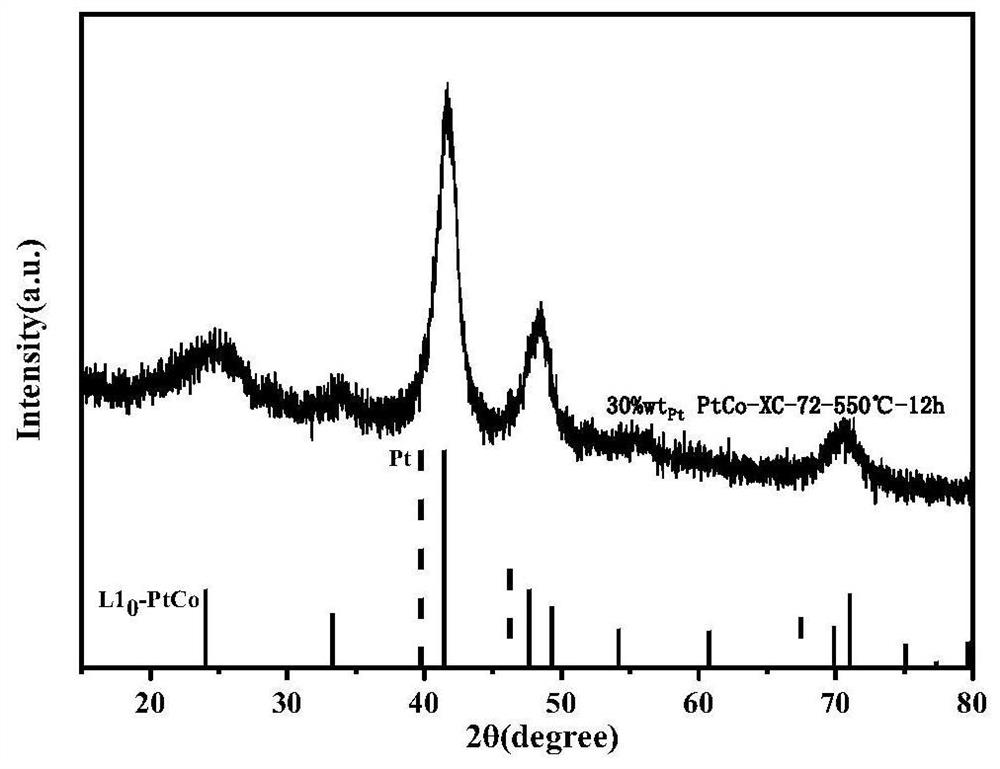
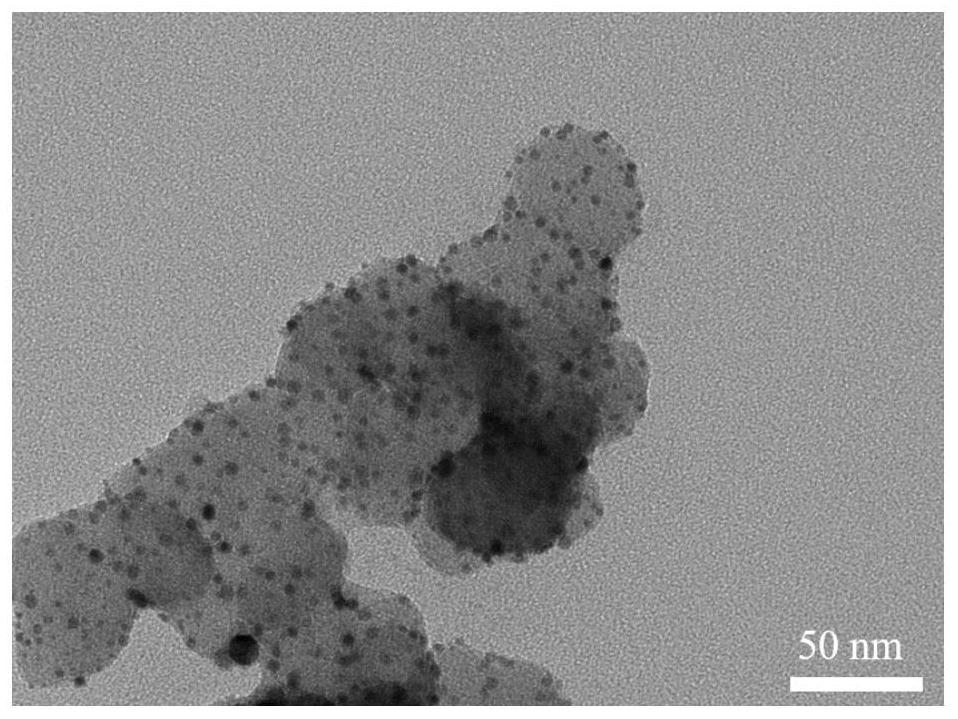
Intermetallic compound catalyst and method for preparing intermetallic compound catalyst by using bimetallic complex
An intermetallic compound, bimetallic complex technology, applied in the field of nanomaterials, can solve the problems of lack of ordered platinum-based alloy nanoparticle size and composition uniformity, decrease of catalyst electrochemically active surface area, and unsuitable for large-scale preparation, etc. To achieve the effect of universality, controllable composition, and high degree of atomic alloying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] a. Disperse 68 μL of thioacetic acid solution into 30 ml of water, then add 85 mg of sodium bicarbonate solution dissolved in 3 ml of water, stir at room temperature for 5 minutes, then add 100 mg of potassium chloroplatinite solution dissolved in 3 ml of water and Dissolve 57mg of cobalt chloride hexahydrate solution in 3ml of water, and continue to stir at room temperature for 24h to obtain a gray precipitate containing a platinum-cobalt double metal complex;
[0045] b. Suction filter and wash the above-mentioned aqueous solution containing the molecules of the platinum-cobalt double metal complex, repeat three times, and then put it into a vacuum drying oven at 65° C. for 6 hours;
[0046]c. Take 28 mg of the above-mentioned completely dried bimetallic complex molecules and 20 mg of carbon black XC-72 and dissolve them in a mixed solvent of 10 ml of tert-butanol and 12 ml of water, and then ultrasonicate for 1 hour in an ultrasonic machine, and transfer the obtained ...
Embodiment 2
[0051] a. Disperse 68 μL of thioacetic acid solution into 30 ml of water, then add 85 mg of sodium bicarbonate solution dissolved in 3 ml of water, stir at room temperature for 5 minutes, then add 100 mg of potassium chloroplatinite solution dissolved in 3 ml of water and Dissolve 57mg of cobalt chloride hexahydrate solution in 3ml of water, and continue to stir at room temperature for 24h to obtain a gray precipitate containing a platinum-cobalt double metal complex;
[0052] b. Suction filter and wash the above-mentioned aqueous solution containing the molecules of the platinum-cobalt double metal complex, repeat three times, and then put it into a vacuum drying oven at 65° C. for 6 hours;
[0053] c. Dissolve 47.4mg of the above-mentioned completely dried bimetallic complex molecules and 20mg of carbon black KJ300 into a mixed solvent of 10ml of tert-butanol and 12ml of water, and then ultrasonicate for 1 hour in an ultrasonic machine, and transfer the obtained homogeneous m...
Embodiment 3
[0058] a. Disperse 68 μL of thioacetic acid solution into 30 ml of water, then add 85 mg of sodium bicarbonate solution dissolved in 3 ml of water, stir at room temperature for 5 minutes, then add 100 mg of potassium chloroplatinite solution dissolved in 3 ml of water and Dissolve 67 mg of ferrous sulfate heptahydrate solution in 3 ml of water, and continue to stir at room temperature for 24 hours to obtain a precipitate containing a platinum-iron double metal complex;
[0059] b. Suction filter and wash the aqueous solution of the molecule containing the platinum-iron double metal complex, repeat three times, and then put it into a vacuum drying oven at 65°C for 6 hours;
[0060] c. Dissolve 27.6mg of the above-mentioned fully dried bimetallic complex molecules and 20mg of carbon black XC-72 into a mixed solvent of 10ml of tert-butanol and 12ml of water, and then ultrasonicate for 1h in an ultrasonic machine, and transfer the obtained homogeneous mixture to In a centrifuge tu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com