Preparation method of cefdinir
A technology of cefdinir and cephalosporin, applied in the field of medicine, can solve the problems of low content, high energy consumption, long reaction time, etc., and achieve the effects of improving product yield, improving quality, and having no toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
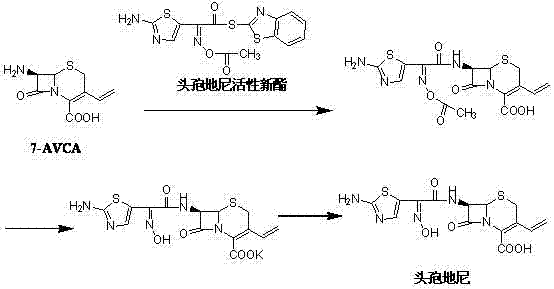
[0034] In a 1000ml four-neck flask, add 170ml tetrahydrofuran, 100ml purified water, stir evenly, at a temperature of 16°C, add 20g (0.0884mol) 7-AVCA, 36g (0.0951mol) cefdinir active new ester, stir evenly, weigh 9.40 g (0.0929mol) of triethylamine was dissolved in 30ml of tetrahydrofuran, and the tetrahydrofuran solution of triethylamine was slowly added dropwise to the four-necked bottle for about 1.5 hours. Extract twice, and the aqueous phase is about 120ml of cefdiniridine solution.
[0035] Control the temperature at 25°C, add 100ml tetrahydrofuran to the prepared cefdinirate solution, and add 13.8g (0.2580mol) NH 4 Cl, then dropwise added 51% K 2 CO 3 Solution 27g, dropwise, add 40g (0.408mol) potassium acetate, with 20%H 2 SO 4 Control the pH to 8.0-8.2, stir for 1 hour, then lower the temperature to 0-5°C and stir for 1 hour, and filter to obtain 40.5 g of a wet product of potassium salt.
[0036] Control the temperature at 25°C, dissolve the prepared potassium ...
Embodiment 2
[0038] In a 1000ml four-necked flask, add 120ml tetrahydrofuran, 150ml purified water, stir evenly, at a temperature of 16°C, add 20g (0.0884mol) 7-AVCA, 36g (0.0951mol) cefdinir active new ester, stir evenly, weigh 14.40 g (0.117mol) of triethylamine was dissolved in 30ml of tetrahydrofuran, and the tetrahydrofuran solution of triethylamine was slowly added dropwise to the four-necked bottle for about 1.5 hours. Extract twice, and the aqueous phase is about 170ml of cefdiniridine solution.
[0039] Control the temperature at 15°C, add 150ml of acetone to the prepared cefdinirate solution, and add 13.8g (0.2580mol) of NH 4 Cl, then dropwise added 51% K 2 CO 3 Solution 27g, dropwise, add 70g (0.714mol) potassium acetate, with 20%H 2 SO 4 Control the pH to 8.0-8.2, stir for 1 hour, then lower the temperature to 0-5°C and stir for 1 hour, filter to obtain 38.7 g of a wet product of potassium salt.
[0040] Control the temperature at 25°C, dissolve the prepared potassium salt...
Embodiment 3
[0042] In a 1000ml four-neck flask, add 170ml tetrahydrofuran, 100ml purified water, stir evenly, at a temperature of 16°C, add 20g (0.0884mol) 7-AVCA, 36g (0.0951mol) cefdinir active new ester, stir evenly, weigh 11.16 g (0.0976mol) of triethylamine was dissolved in 30ml of tetrahydrofuran, and the tetrahydrofuran solution of triethylamine was slowly added dropwise to the four-necked bottle for about 1.5 hours. Extract twice, and the aqueous phase is about 120ml of cefdiniridine solution.
[0043] Control the temperature at 25°C, add 40ml of tetrahydrofuran to the prepared cefdinirate solution, and add 13.8g (0.2580mol) of NH 4 Cl, then dropwise added 51% K 2 CO 3 Solution 27g, dropwise, add 10g (0.102mol) potassium acetate, with 20%H 2 SO 4 Control the pH to 8.0-8.2, stir for 1 hour, then lower the temperature to 0-5°C and stir for 1 hour, filter to obtain 39.8 g of a wet product of potassium salt.
[0044] Control the temperature at 25°C, dissolve the obtained potassiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com