Edaravone A-type crystal and preparation method thereof
A technology of edaravone and crystal, which is applied in the field of new crystal form of 1-phenyl-3-methyl-5-pyrazolone and its preparation field, can solve the problem of few crystal form patent reports, low yield, Pollution and other problems, to reduce the risk, improve the purity, easy to store the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of Edaravone type A crystal
[0025] Add 96ml ethanol, 16ml n-hexane, and 32.2g of Edaravone crude product with HPLC purity of 99.0% by ethanol: n-hexane 6:1 ratio in the reaction flask, keep the stirring speed of 300 rev / min, control the solution to be stable to Reflux started at 65°C and the solid dissolved. After stirring for about 30 minutes, start to cool down, increase the stirring speed to 500 rpm, solids start to precipitate at 45°C, stir and crystallize at 10°C for 1 hour, filter, wash the filter cake with ethanol and n-hexane, and dry under reduced pressure at 90°C for 9 hours. 25.6 g of white crystalline product was obtained with a purity of 99.9% and a yield of 79.5%.
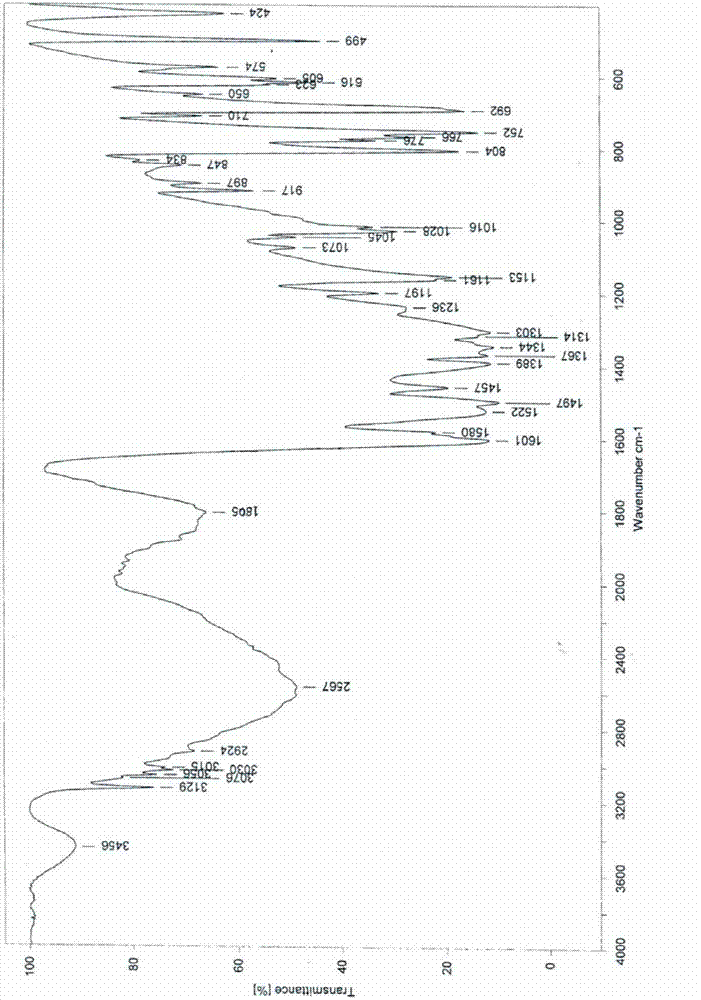
[0026] Infrared (IR) spectrum data are: 3456, 3129, 1805, 1601, 1580, 1522, 1497, 1457, 1389, 1367, 1344, 1314, 1303, 1236, 1197, 1153, 1045, 1028, 1016, 917, 804, 766 and 752cm -1 .
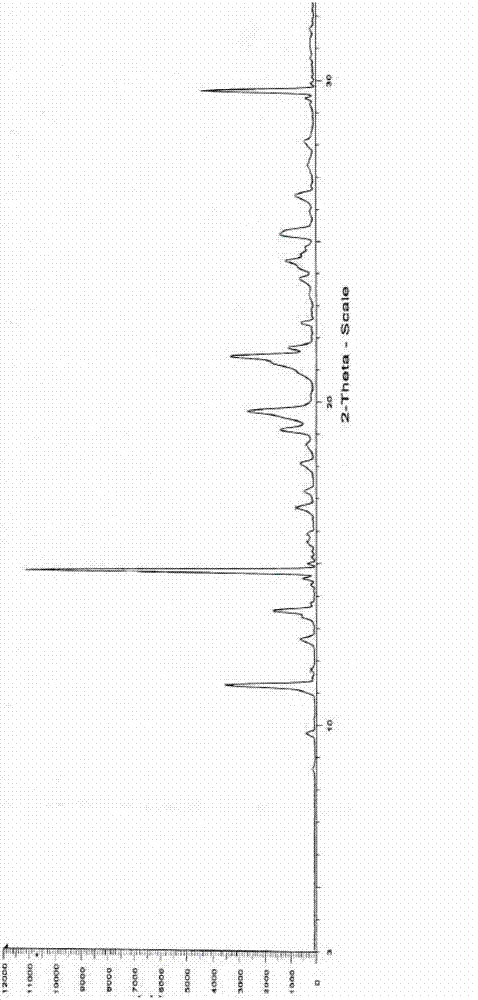
[0027] Measure under the following experimental conditions to obtain the powde...
Embodiment 2
[0032] Embodiment 2: Preparation of Edaravone type A crystal
[0033] Add 72ml ethanol, 12ml petroleum ether, and 25.0g of Edaravone crude product with HPLC purity of 99.0% by ethanol: the ratio of petroleum ether 6:1 in the reaction bottle, keep the stirring speed of 300 rev / min, control the solution to be stable to Reflux started at 70°C and the solid dissolved. After stirring for about 30 minutes, start to cool down, increase the stirring speed to 500 rpm, solids start to precipitate at 40°C, stir and crystallize at 10°C for 1 hour, filter, wash the filter cake with a mixture of ethanol and petroleum ether, and dry under reduced pressure at 90°C After 9 hours, 19.0 g of a white crystalline product was obtained with a purity of 99.8% and a yield of 76.2%. Infrared (IR) spectrum and X-powder diffraction pattern are the same as in Example 1.
Embodiment 3
[0034] Embodiment 3: Preparation of Edaravone type A crystal
[0035] Add 150ml of ethanol, 25ml of ether, and 50.0g of crude Edaravone with an HPLC purity of 99.0% in the reaction flask according to the ratio of ethanol: ether 6:1, keep the stirring speed at 300 rpm, and control the solution to stabilize at 70°C Reflux was initiated and the solid dissolved. After stirring for about 30 minutes, start to lower the temperature, increase the stirring speed to 500 rpm, and start to precipitate solids at 40°C, stir and crystallize at 10°C for 1 hour, filter, wash the filter cake with a mixture of ethanol and ether, and dry under reduced pressure at 90°C for 9 hours, 38.4 g of a white crystalline product was obtained, with a purity of 99.8% and a yield of 76.8%. Infrared (IR) spectrum and X-powder diffraction pattern are the same as in Example 1.
[0036] EFFECT EXAMPLES Properties of Edaravone Form A
[0037] 1. Solubility
[0038] Accurately weigh an appropriate amount of Edar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com