Quality control method for edaravone and edaravone-containing preparation
A quality control method, edaravone technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems affecting drug efficacy and activity, increase adverse drug reactions, difficulties in production and clinical use, etc., and achieve accurate quantitative determination of impurities Effect
Inactive Publication Date: 2011-02-09
YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD +2
View PDF1 Cites 10 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0017] Embodiment 1: Determination of the content of impurity Edaravone dimer in Edaravone by high performance liquid chromatography
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention discloses a quality control method for edaravone and an edaravone-containing preparation. In the method, an impurity, namely, 2,2',4,4'-tetrahydrophthalic anhydride-5,5'-dimethyl-2,2'-diphenyl-[4,4'-di-3H-parazole]-3,3'-dione in the edaravone and an edaravone preparation is determined quantitatively by photoelectric technology. By the technical scheme of the invention, the qualities of the edaravone and an edaravone-containing medicament preparation can be evaluated and controlled more reliably and accurately.
Description
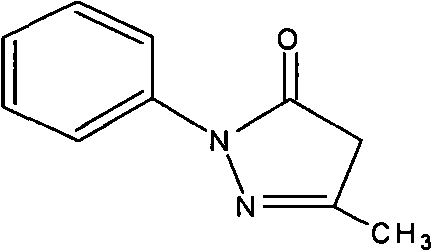
Technical field: [0001] The invention relates to a quality control method of a medicine and its preparation, in particular to a quality control method of a cranial nerve protective agent edaravone and a preparation containing edaravone. Background technique: [0002] Edaravone is one of pyrazolone derivatives, its chemical name is 3-methyl-1-phenyl-2-pyrazolin-5-one, molecular formula C 10 h 10 N 2 O, the chemical structural formula is as follows: [0003] [0004] Edaravone was first developed by Japan's Mitsubishi Tokyo Pharmaceutical Company, and was approved for clinical use by the Ministry of Medical Labor and Welfare in Japan on April 4, 2001, with a trade name of Radicut. The dosage form of the drug listed in Japan is water injection, the specification is 30mg / 20ml, and the excipients are sodium bisulfite and cysteine hydrochloride. The usual dosage is 30mg each time, twice a day, dissolved in normal saline or other diluents Intravenous infusion, generally us...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): G01N30/90G01N30/02G01N30/36
Inventor 薛明阮锦满史美耿王永毅石海云钱世祥朱静娟
Owner YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com