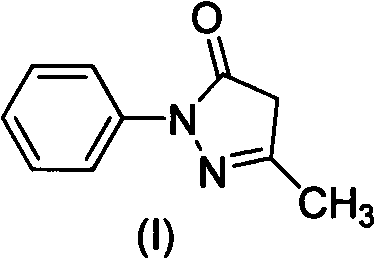
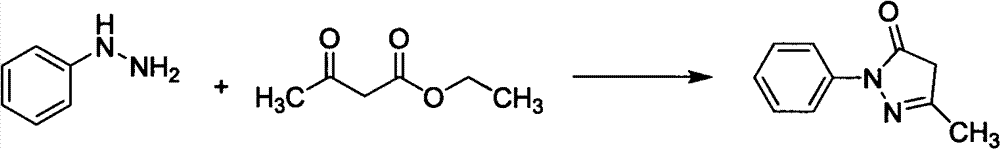
Preparation method for edaravone
A technology of edaravone and protonic acid, which is applied in the field of preparation of brain protective agent edaravone, can solve the problems of unfavorable industrial production, cumbersome operation process, long reaction time, etc., and achieve the reduction of heating and cooling steps and safe operation Convenience and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add phenylhydrazine (490ml, 4.98mol) dropwise into the mixture of ethyl acetoacetate (636ml, 4.98mol) and acetic acid (28.5ml, 0.498mol), stir, control the rate of addition, keep the reaction temperature at 40-45°C, and complete the dropwise addition Afterwards, the reaction was continued for 20 min, water (2000 ml) was added, solids were precipitated, filtered, and dried to obtain 840 g of crude product Edaravone, with a yield of 96.8%, and a product purity of 98.6% through HPLC analysis.
[0034] mp: 127.1-127.9°C.
Embodiment 2
[0036] Add phenylhydrazine (490ml, 4.98mol) dropwise into the mixture of ethyl acetoacetate (636ml, 4.98mol) and acetic acid (285ml, 4.98mol), stir, control the rate of addition, and keep the reaction temperature at 40-45°C. , continued to react for 20min, added water (2000ml), precipitated solid, filtered, and dried to obtain crude product Edaravone 833g, yield 96.0%, product purity 98.8% through HPLC analysis.
[0037] mp: 127.2-128.1°C.
Embodiment 3
[0039]Phenylhydrazine (490ml, 4.98mol) was added dropwise into a mixture of ethyl acetoacetate (636ml, 4.98mol) and hydrochloric acid (500ml, 5.98mol), stirred, and the rate of addition was controlled to keep the reaction temperature at 70°C. After the addition was complete, continue After reacting for 50 minutes, water (2000ml) was added to precipitate a solid, which was filtered and dried to obtain 810g of crude Edaravone with a yield of 93.3%. The purity of the product was analyzed by HPLC to be 97.2%.
[0040] mp: 127.0-128.0°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com