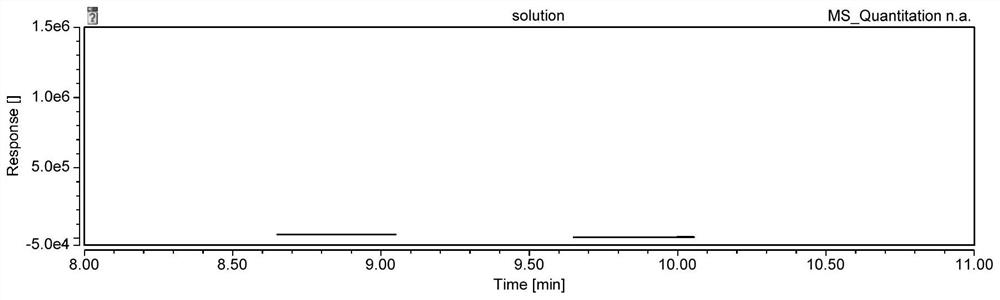
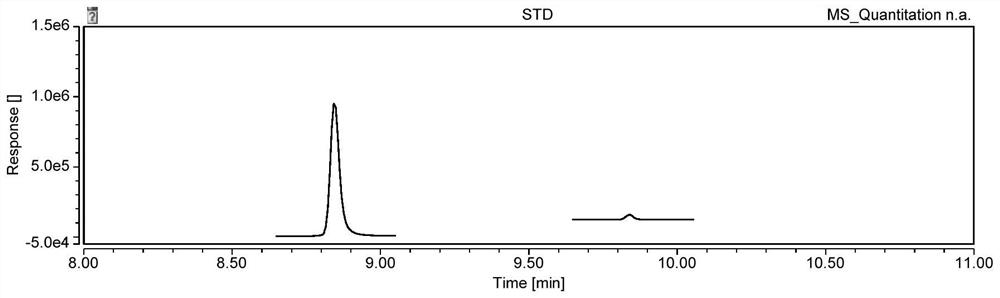
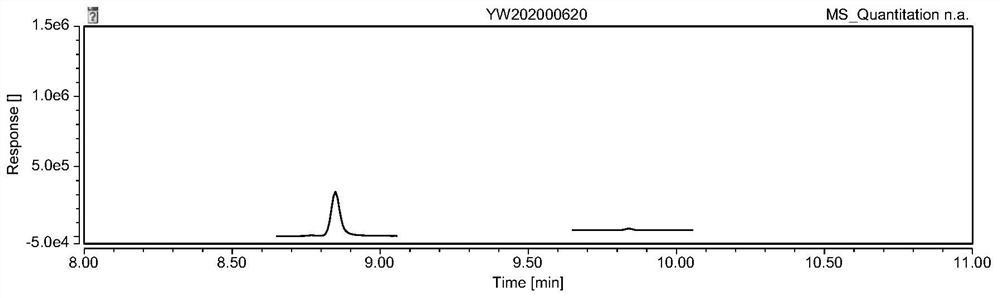
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
52 results about "Formic acid ethyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
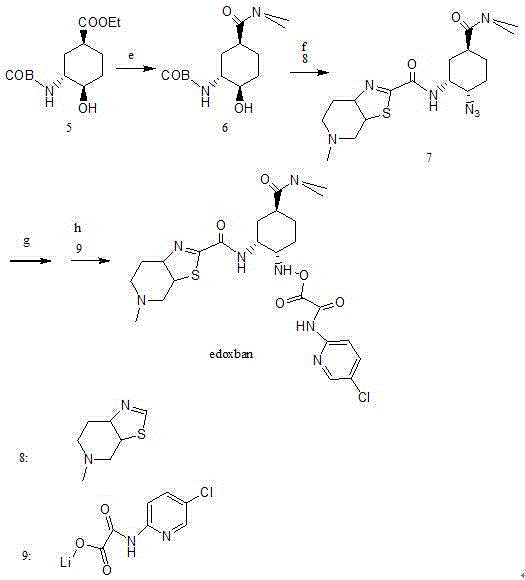
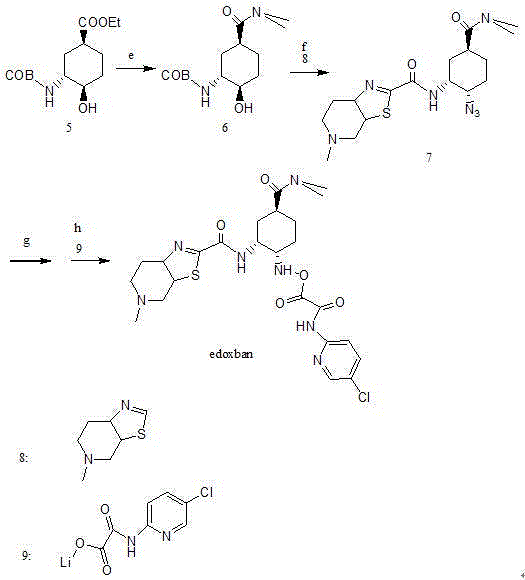
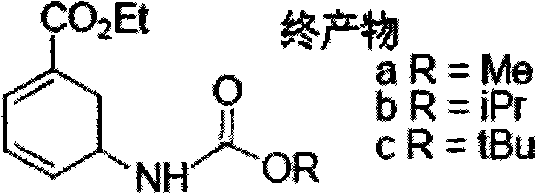
Edoxaban intermediate and preparation method thereof
InactiveCN105198776AStable and reliable quantityHigh yieldCarbamic acid derivatives preparationOrganic compound preparationPtru catalystFormic acid ethyl ester
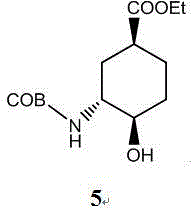
The invention relates to an edoxaban intermediate and a preparation method thereof. The preparation method of the edoxaban intermediate comprises steps as follows: (a), (1s)-3- cyclohexene-1-formic acid is taken as a raw material, and a compound 2 is generated in the presence of a catalyst, a halogen and a weak base; (b), the compound 2 generates a compound 3 under the action of strong base in an absolute ethanol solution; (c), the compound 3 generates a compound 4 in an ammonia and ethanol solution; (d), the compound 4 reacts with a protecting group of amino under the action of a catalyst, and a compound 5, (1S,3R,4R)-3-[(t-butyloxycarboryl)amino]-4-hydroxycyclohexyl ethyl formate, is generated. Raw materials and reagents required in the synthesis method of the edoxaban intermediate are easy to obtain, the yield is high, the cost is low, reaction conditions are mild, three wastes are relatively fewer, and the quality of the product is stable and reliable.
Owner:天津药物研究院药业有限责任公司
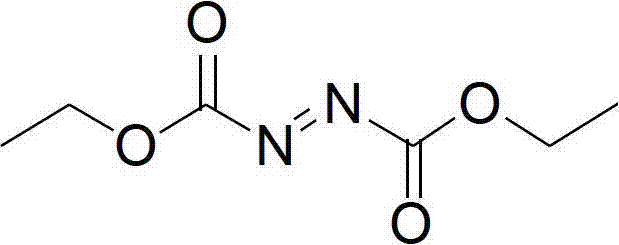
Synthesis method of diethyl azodicarboxylate and intermediate of diethyl azodicarboxylate
ActiveCN102898328ANo pollution in the processIn line with the concept of modern green chemistryHydrazide preparationDiisopropyl azodicarboxylateDistillation
The invention discloses a synthesis method of diethyl azodicarboxylate. The synthesis method comprises the following steps that (1) under the effect of sodium ethoxide, diethyl carbonate and ethyl carbazate are heated for reaction for 1 to 6 hours, the pH of solution is regulated to 3 to 8, white crystals are separated out, the recrystallization is carried out, and hydrogenated diethyl azodicarboxylate is obtained; and (2) the hydrogenated diethyl azodicarboxylate is added at minus 15 DEG C to 45 DEG C, in the acid solution, bromine or hydrobromic acid or sodium bromide and potassium bromide are used as catalysts, excessive hydrogen peroxide is dripped, the reaction is carried out for 1 to 10 hours, the extraction is carried out, organic solvents are removed through distillation, and saffron diethyl azodicarboxylate is obtained. The invention also provides a hydrogenated diethyl azodicarboxylate intermediate and a synthesis method of the hydrogenated diethyl azodicarboxylate intermediate. The synthesis method has the advantages that diethyl carbonate is used as raw materials, cleanness and environment protection are realized, no pollution exists, raw materials can be cyclically utilized, the economy is better, the operation is simple, the reaction temperature range is wide, the reaction is stable, the energy consumption is low, the yield is high, and the industrial production is favorably realized.
Owner:SHANDONG NORMAL UNIV
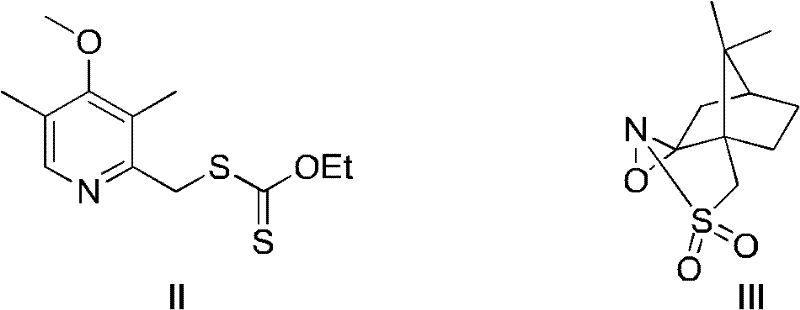
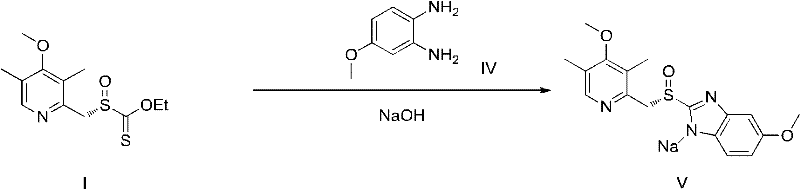
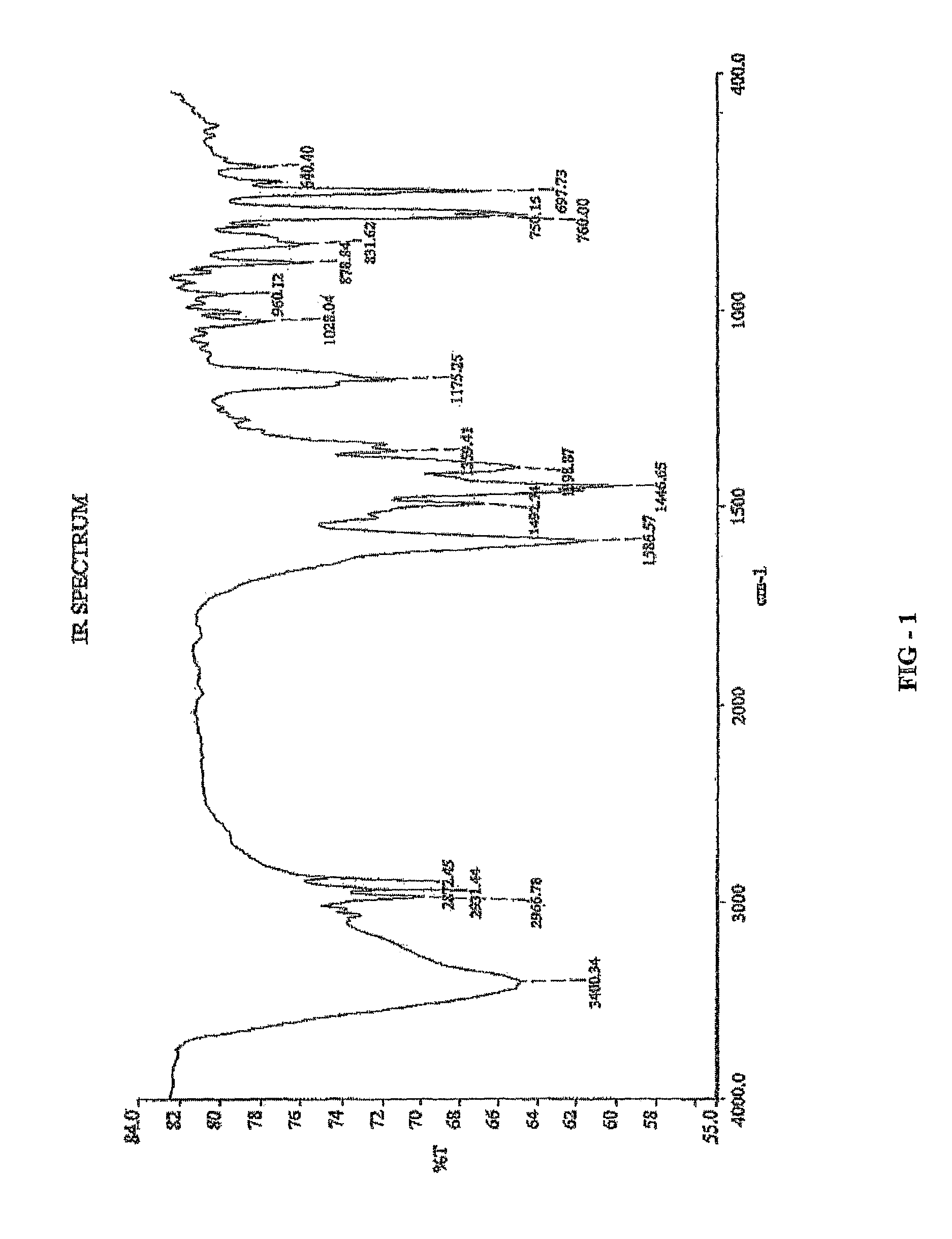
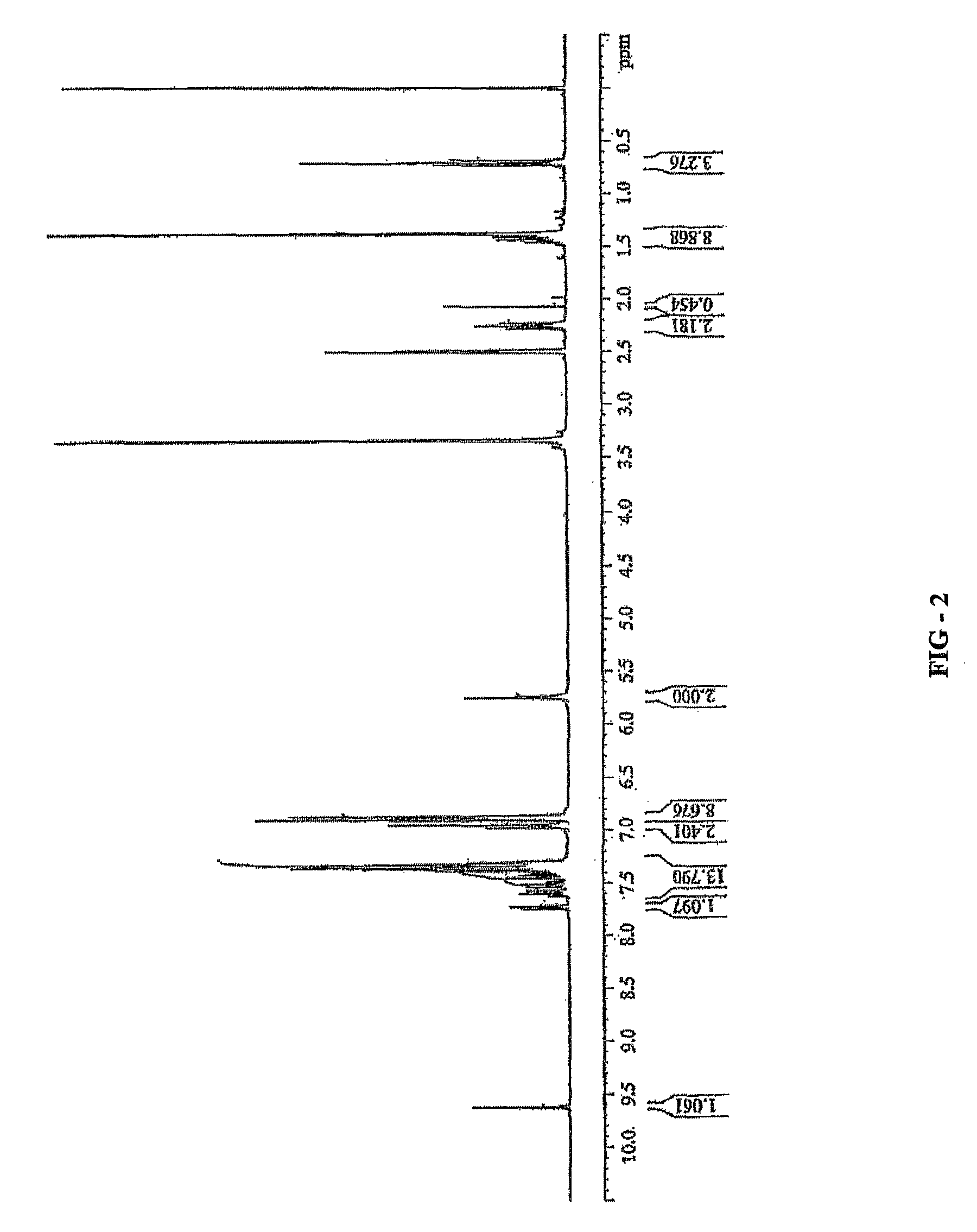
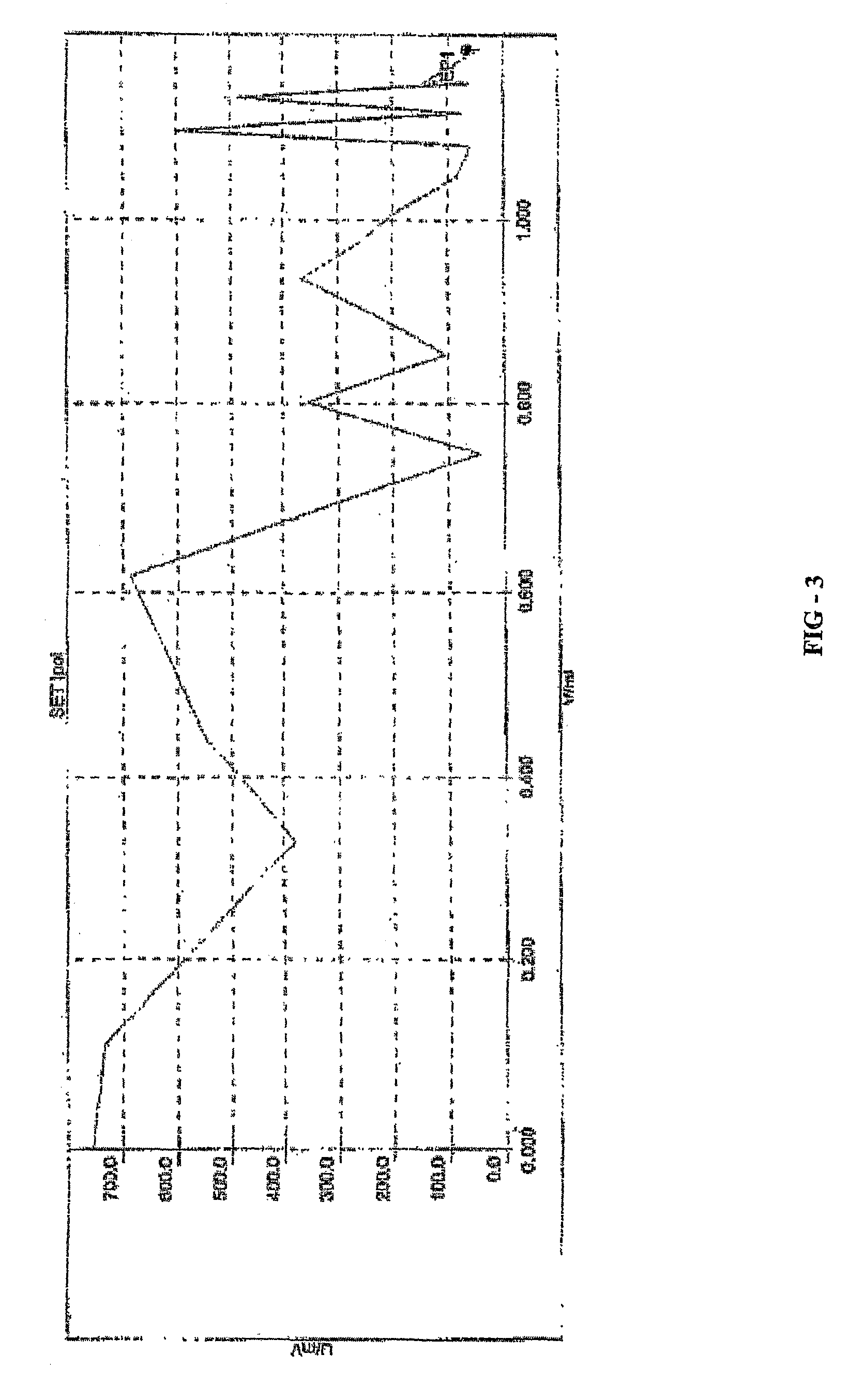
Novel chiral sulfoxide compound and method for preparing esomeprazole by using novel chiral sulfoxide compound
The invention discloses a (S)-(((4-methoxy-3,5-dimethyl pyridine-2-yl)methyl)sulfinyl) ethyl thioformate compound, a preparation method of the compound and a process for preparing esomeprazole by using the compound. The compound and the preparation method have the remarkable advantages that: raw materials for synthesis are cheap and can be easily obtained; the reaction conditions are moderate; the yield of the prepared esomeprazole is high; the optical purity is high; the operation is safe; the environment is slightly polluted; and the compound is more suitable for industrial large-scale production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
Process for the preparation of olmesartan medoxomil
Owner:MYLAN LAB
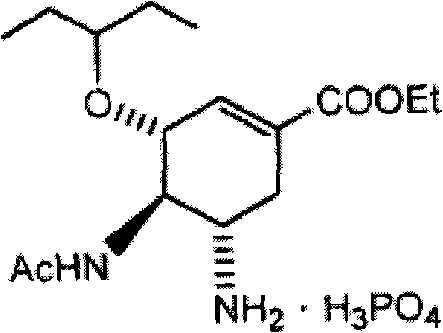
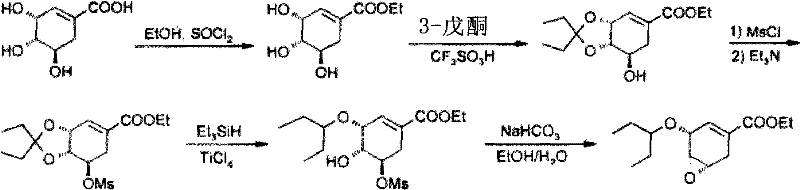
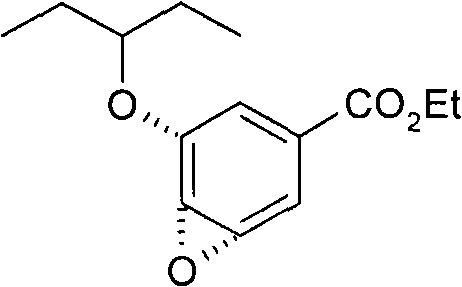
Process for the preparation of (3r,4r,5s)-4,5-epoxy-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid ethyl ester
InactiveCN102267961ALow raw material costAvoid it happening againOrganic chemistrySolventCarboxylate
The invention discloses a method for preparing ethyl (3R,4R,5S)-4,5-epoxy-3-(1-ethyl-propoxy)-1-cyclohexene-1-formate. The preparation method disclosed by the invention comprises the following steps: (a) dissolving ethyl (3R,4R,5R)-3-(1-ethyl-propoxy)-4-hydroxy-5-O-methyl-sulfonyl-1-cyclohexene-1-formate in an alcohol solvent, and carrying out elimination reaction under the effect of ammonia water; and (b) collecting ethyl (3R,4R,5S)-4,5-epoxy-3-(1-ethyl-propoxy)-1-cyclohexene-1-formate from the reaction solution. By using the method disclosed by the invention, the product yield reaches more than 90%, the product purity measured by HPLC (high-performance liquid chromatography) reaches more than 99.5%, and the individual impurity content in the product is less than 0.1%; and the method is suitable for industrial mass production.
Owner:SHANGHAI ACEBRIGHT PHARMA GRP +2
One-pot method for preparing crucial intermediate in oseltamivirphosphate synthesizing reaction
InactiveCN102464596AShort reaction pathShort synthesis cycleCarbamic acid derivatives preparationOrganic compound preparationChemistryHydroquinone Compound
The invention discloses a one-pot method for preparing a crucial intermediate in oseltamivirphosphate synthesizing reaction. The method is characterized in that: 1,3-butadiene and ethyl propiolic acid ethyl ester are adopted as raw materials, alcohol and isocyanate / salt are adopted as main reactants, DBU is adopted as a catalyst, and no separation process is required before a finished product is obtained, such that the one-pot method is realized. The method mainly comprises steps that: 1, 1,3-butadiene is cooled to a temperature of -78 DEG C; ethyl propiolic acid ethyl ester and hydroquinone are added to 1,3-butadiene, and the mixture is stirred for 3 days under a temperature of 110 DEG C; the mixture is subject to vacuum distillation, such that 1-ethyl formate-1,4-diene cyclohexane is obtained; 2, I2 is added to a solution of 1-ethyl formate-1,4-diene cyclohexane and AgOCN; the mixture is heated to a temperature of 35-40 DEG C within 4 hours, such that a mixture A is obtained; alcohol and waterless HCl are added to the mixture A, and the mixture is stirred for 6-8 hours under a temperature of 35-40 DEG C, such that a mixture B is obtained; DBU is added to the mixture B, and the mixture is subject to a reaction over night under a temperature of 35-40 DEG C; the mixture is purified, such that the finished product is obtained; or DBU is added to the mixture A, the mixture is subject to a reaction over night under a temperature of 35-40 DEG C, and the mixture is purified, such that the finished product is obtained. Compared to existing technologies, the method is substantially advantaged in that: initial raw materials are cheap and easy to obtain, the reaction route is short, the synthesis period is shortened, the reaction method is improved into a one-pot method, the separation and purification processes are simple, the yield is high, the reaction efficiency is high, and the method has certain potential to be used in large-scaled productions.
Owner:NANKAI UNIV
Method for synthesis of tyrosine derivative
InactiveCN102659619AImprove product qualityOrganic compound preparationCarboxylic acid amides preparationEthyl chloroformatePhenacyl
The invention discloses a method for synthesis of a tyrosine derivative. The method is a novel method for preparing the tyrosine derivative N-benzoyl-L-tyrosyl-di-n-propylamine. The method mainly solves the problem that the existing synthesis method has a low yield and low product purity. The method realizes the preparation of N-benzoyl-L-tyrosyl-di-n-propylamine by a two-step synthesis technology and comprises the following steps that 1, raw materials of L-tyrosine and benzoyl chloride undergo a reaction in the presence of an inorganic alkali aqueous solution and O,N-(2-benzoyl)-L-tyrosine crystallizes in an acetone aqueous solution; 2, the O,N-(2-benzoyl)-L-tyrosine, triethylamine, ethyl chloroformate and isopropamide are synthesized into O,N-(2-benzoyl)-L-tyrosyl-diisopropylamine in the presence of ethyl acetate; and 3, the O,N-(2-benzoyl)-L-tyrosyl-diisopropylamine is subjected to ethyl acetate extraction and condensation; then benzoyl of an oxy group is removed in an alcohol under alkaline conditions; and acidity adjustment is carried out in an alcohol aqueous solution so that N-benzoyl-L-tyrosyl-di-n-propylamine is obtained. The method is mainly used for preparation of an intermediate of a drug tiropramide hydrochloride from tyrosine as an initial raw material.
Owner:SHANGHAI JUNJIE BIOCHEM TECH +1
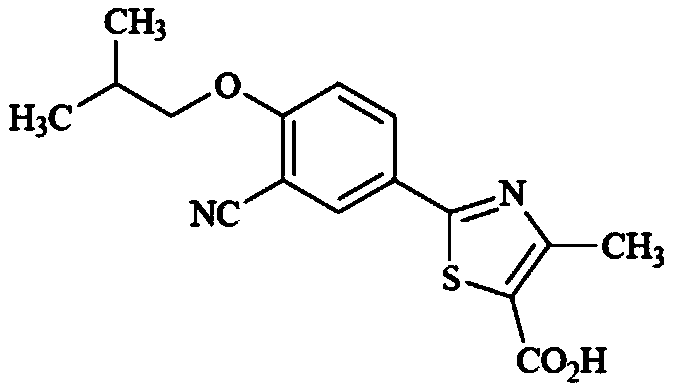
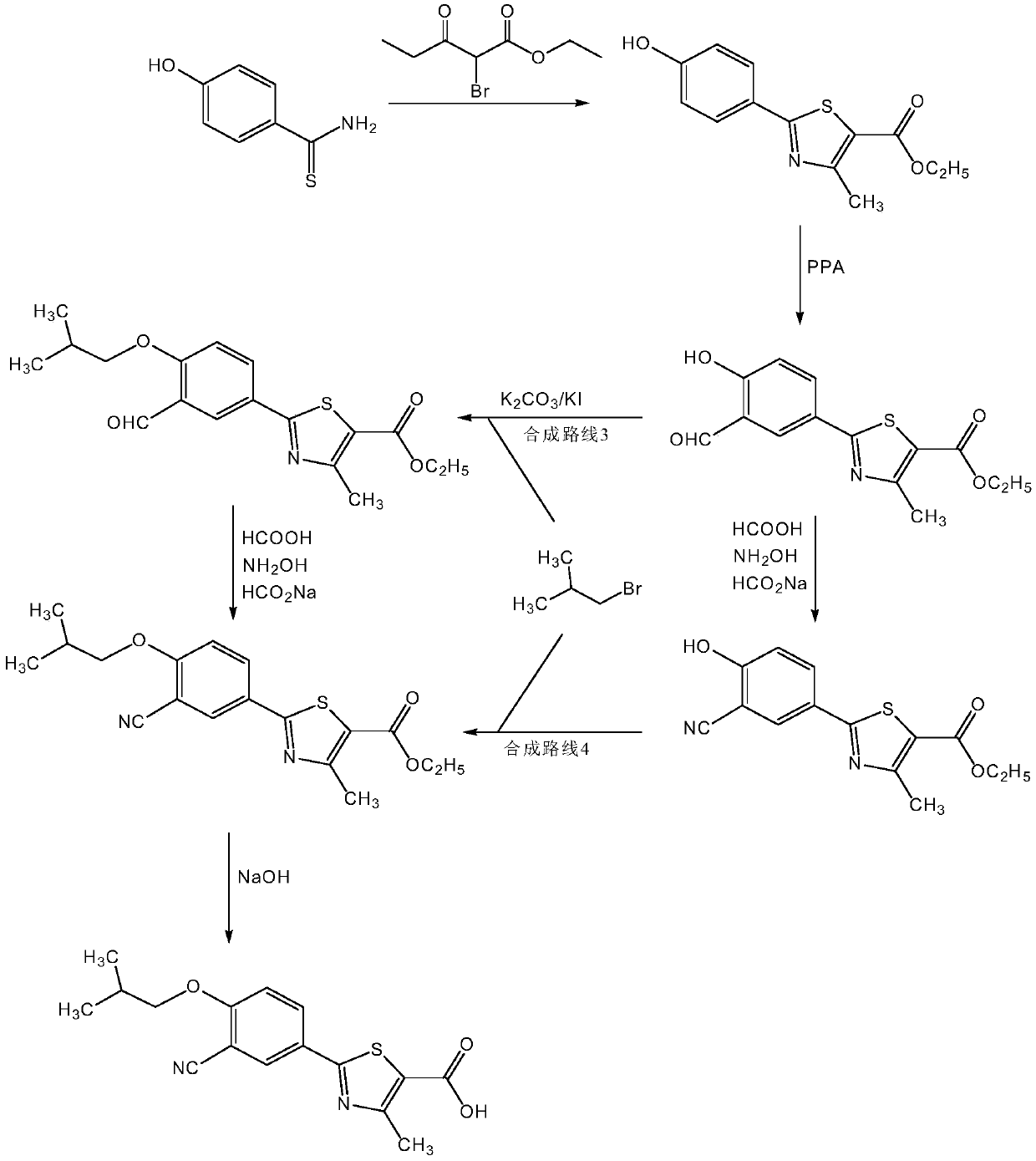
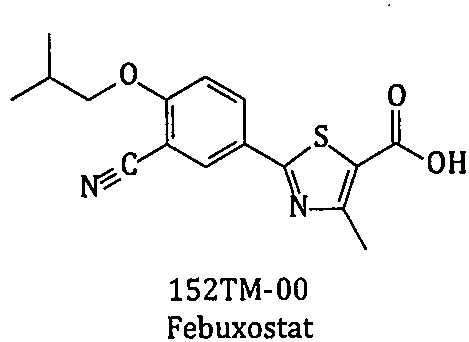
Method for synthesizing febuxostat and intermediate thereof
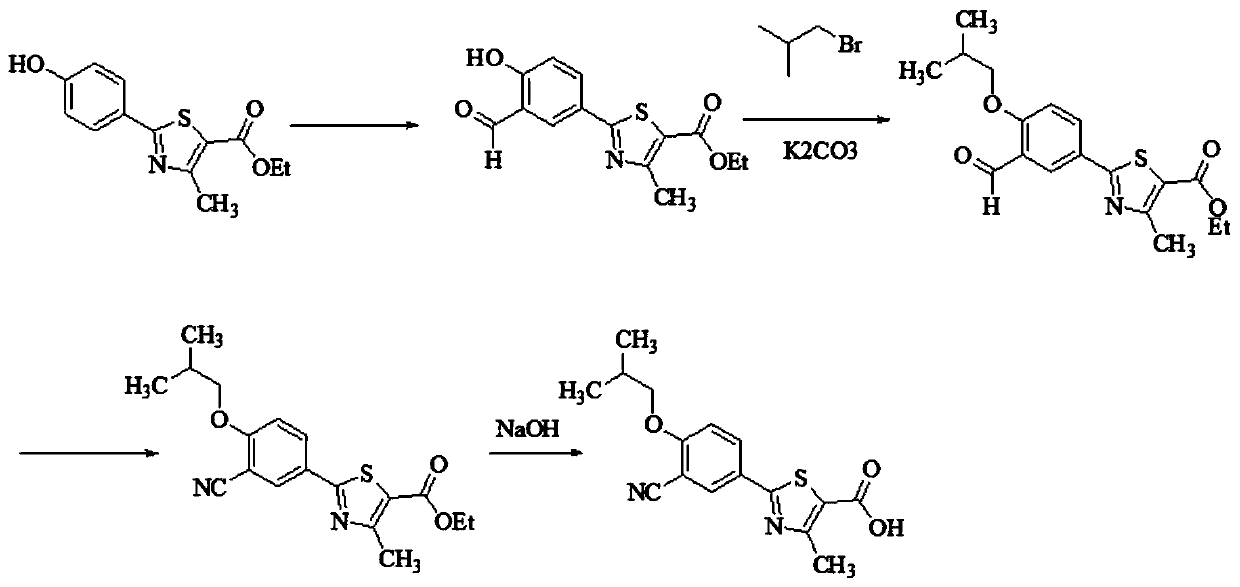
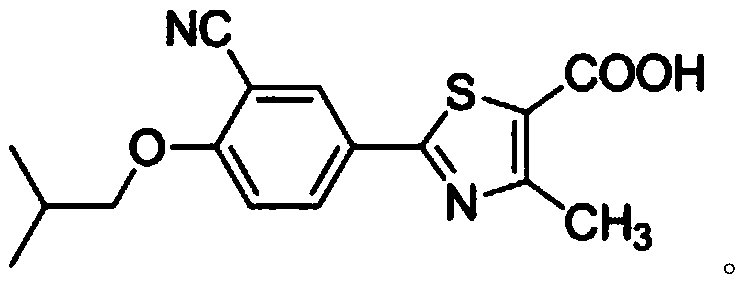
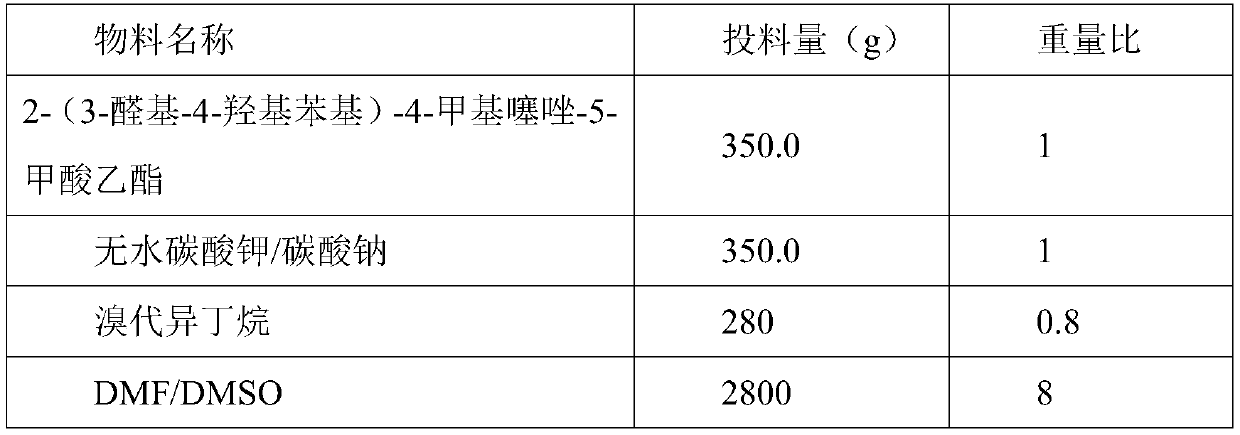
The invention relates to a method for synthesizing febuxostat and an intermediate thereof, specifically a method for synthesizing 2-(3-formyl-4-isobutoxy-phenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester. The method comprises the following steps: preparing 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-carboxylate; enabling the product obtained in the step (a) to react in DMF (Dimethyl Formamide) in the presence of potassium carbonate and bromo-isobutane, adding water and ethyl acetate for extraction, concentrating to obtain an organic layer, and recrystallizing with DMF to obtain 2-(3-formyl-4-isobutoxy-phenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester. The invention also relates to 2-(3-formyl-4-isobutoxy-phenyl)-4-methyl-thiazole-5-carboxylic acid ethyl ester and 2-(3-formyl-4-hydroxyphenyl)-4-methyl-5-thiazoleethyl formate and application thereof to the preparation of febuxostat. The method of the invention has excellent performance.
Owner:HANGZHOU ZHUYANGXIN PHARMA
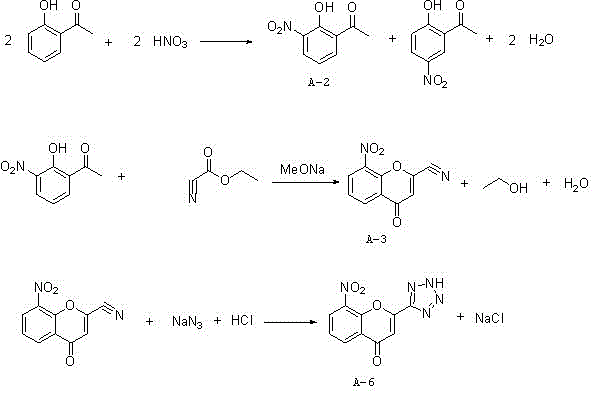
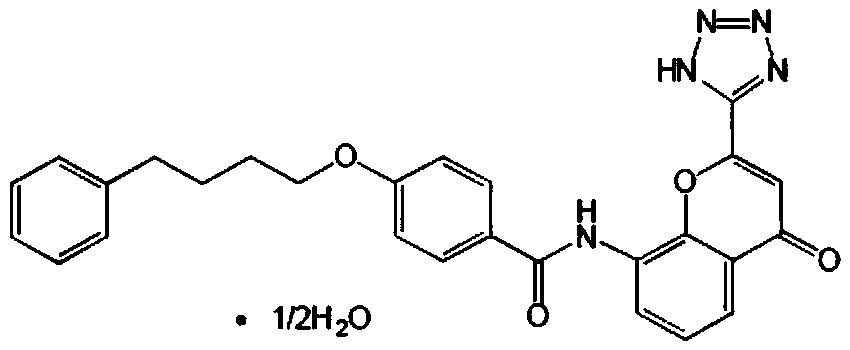
Synthesis method of 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran
ActiveCN103980257ASimple operation processThe reaction steps are simpleOrganic chemistryChlorethoxyfosNitration
The invention relates to a synthesis method of 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran. The method comprises the following steps: firstly nitrifying hydroxyacetophenone taken as a starting material with a nitric acid so as to generate a mixture of 2-hydroxy-3-nitroacetophenone and 2-hydroxy-5-nitroacetophenone; salifying the mixture by using an inorganic base, carrying out separation and purification and then hydrolyzing with an acid so as to obtain 2-hydroxy-3-nitroacetophenone (A-2); carrying out a cyclization reaction on the 2-hydroxy-3-nitroacetophenone (A-2) and ethyl cyanoformate so as to generate 8-nirto-2-cyano-4-oxo-4H-1-benzopyran (A-3); and finally, reacting with sodium azide so as to generate the 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran (A-6). According to the method, phosphorus oxychloride with an application risk and large pollution and an ammonia gas with strong stimulating smell are not used, so that the production is safe and environment-friendly; and the generation of waste gases is reduced at the same time, so that the synthesis process is shortened. Thus, the operation is simplified; the production cost is lowered; and the 8-nitro-2-tetrazol-5-yl-4-oxo-4H-1-benzopyran is high in purity.
Owner:SUZHOU KAIYUAN MINSHENG SCI & TECH CORP
Preparation process of ethyl chromane-4-formate
The invention relates to a preparation process of ethyl chromane-4-formate. The preparation process is characterized by comprising the following steps of: carrying out Friedel-Crafts acylation reaction and cyclization, addition reaction, hydrolysis reaction and esterification reaction, wherein, in the Friedel-Crafts acylation reaction and cyclization, the Friedel-Crafts acylation reaction is carried out in the presence of anhydrous aluminium trichloride by using a compound of formula 1 and 3-chloropropionylchloride as initial raw materials, then carrying out cyclization on an obtained product and 10% NaOH to obtain a compound of formula 2; in the addition reaction, the addition reaction is carried out on the compound of formula 2 and trimethylsilyl cyanide in the presence of zinc iodide to obtain a compound of formula 3; in the hydrolysis reaction, the compound of formula 3 is hydrolyzed under the conditions of stannous chloride dihydrate, concentrated hydrochloric acid and acetic acid to obtain a compound of formula 4; and in the esterification reaction, the compound of formula 4 is esterified in the presence of concentrated sulfuric acid to obtain a compound of formula 5. According to the preparation process, expensive trifluoromethanesulfonic acid is replaced with cheap anhydrous aluminium trichloride in the Friedel-Crafts acylation step, and the yield is improved, so that the cost of preparing the compound is greatly reduced, and the high-quality product is obtained; and compared with the traditional method, the preparation process has obvious advantages.
Owner:苏州莱克施德药业有限公司
Synthesis process of febuxostat
PendingCN111499593AOperational securitySuitable for industrial productionOrganic chemistryFormateBiochemical engineering
The invention discloses a synthesis process of febuxostat. The synthesis process comprises the following steps: taking ethyl 2-(4-hydroxyphenyl)-4-methylthiazole-5-carboxylate as a raw material to prepare ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5- formate; preparing ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-formate from the ethyl 2-(3-formyl-4-hydroxyphenyl)-4-methylthiazole-5-formate; preparing ethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-formate from ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methylthiazole-5-formate; preparing a crude febuxostat product fromethyl 2-(3-cyano-4-isobutoxyphenyl)-4-methylthiazole-5-formate; and specifically crystallizing the febuxostat crude product to prepare a febuxostat pure product, wherein the febuxostat pure product isa target product. The method solves the problems that an original synthesis route is high in operation risk and not suitable for industrial production.
Owner:EMEISHAN HONGSHENG PHARMA
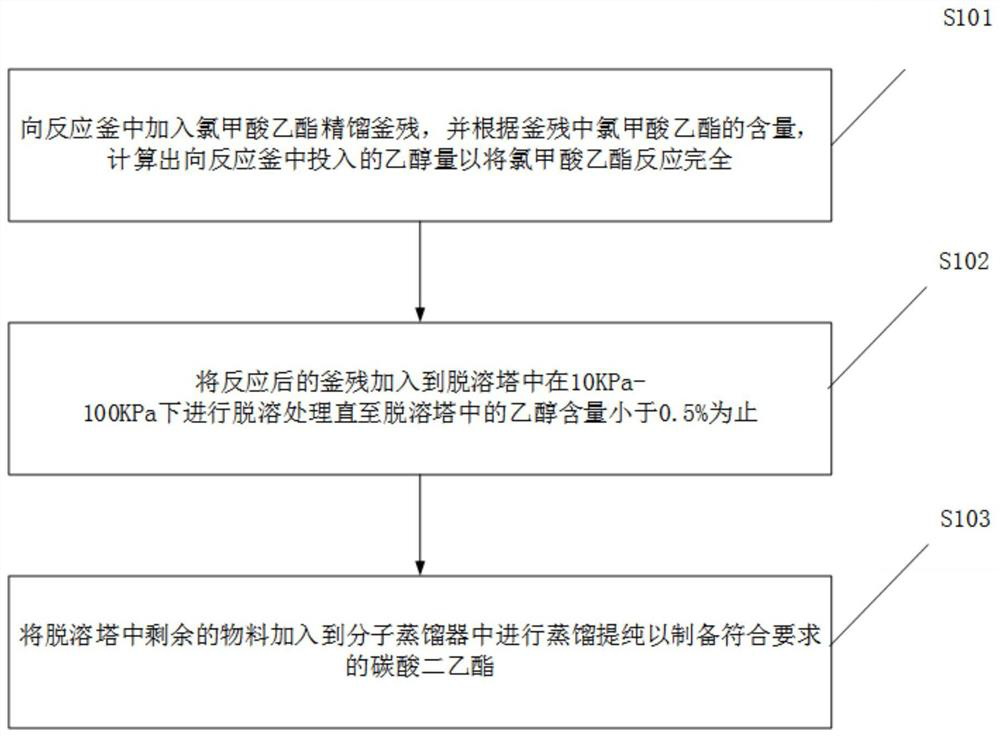
Treatment method of ethyl chloroformate rectifying still residues
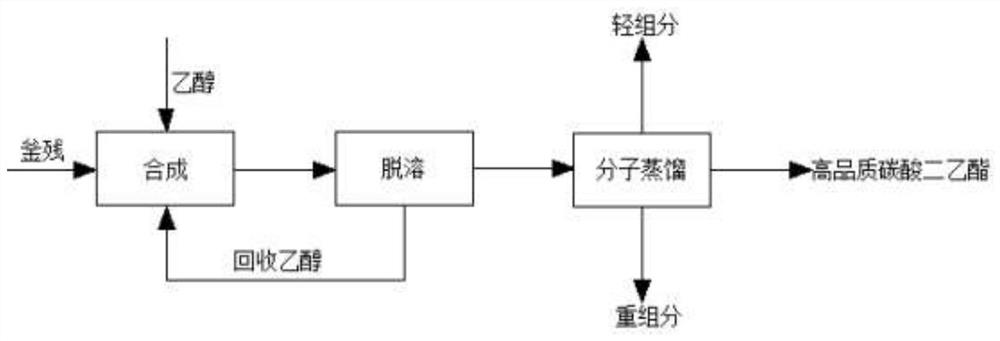
PendingCN113698297AEasy to prepareIncrease contentOrganic compound preparationCarbonic/haloformic acid esters purification/separationEthyl chloroformateDistillation
The invention discloses a method for treating ethyl chloroformate rectifying still residues. The ethyl chloroformate rectifying still residues are further reacted with ethanol, so that ethyl chloroformate in the still residues can be basically reacted completely, the content of diethyl carbonate is increased, the components of the still residues are relatively single, and preparation of high-quality diethyl carbonate is facilitated. The residues subjected to ethanol treatment are subjected to continuous molecular distillation after desolvation and ethanol removal, the system temperature is low, the retention time is short, the heating time of diethyl carbonate is short, and the chromaticity, content, stability and the like of the product are remarkably superior to those of a traditional rectification process. The ethyl chloroformate rectifying still residues are subjected to effective resourceful treatment, high-quality diethyl carbonate is obtained, the amount of three wastes is greatly reduced, and the added value of the product is improved.
Owner:NINGXIA RUITAI TECH +1
New preparation method of febuxostat intermediate
ActiveCN110790720AReduce the molar amountReduce energy consumptionSulfonic acid esters preparationBulk chemical productionHydroxylamine HydrochlorideFebuxostat
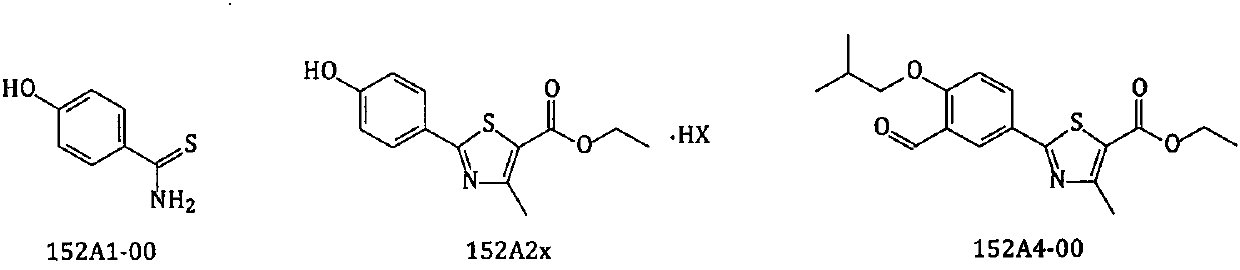
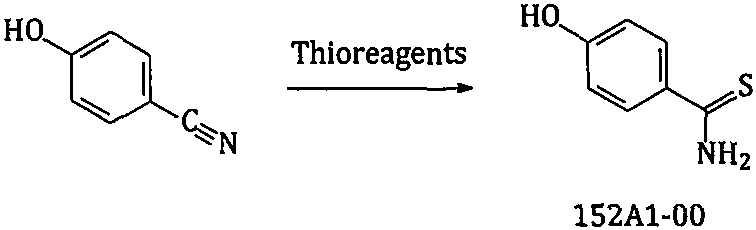
The invention relates to a new preparation method of a febuxostat intermediate. The method includes: taking cheap 4-hydroxybenzaldehyde as an initial raw material, firstly preparing aldoxime from 4-hydroxybenzaldehyde and hydroxylamine hydrochloride, then adding a corresponding thio reagent, and preparing a compound 4-hydroxythiobenzamide (152A1-00) by Beckmann rearrangement reaction; utilizing one-pot process, adopting cheap 4-hydroxybenzaldehyde as an initial raw material, carrying out a series of reactions, and then performing cyclization with 2-halogenated ethyl acetoacetate to obtain ethyl 2-(4-hydroxyphenyl)-4-methyl-5-thiazolecarboxylate or different salt forms (152A2x) thereof; and using isobutyl sulfonate (152H1x) with more easily controllable quality to replace bromo-isobutane soas to prepare ethyl 2-(3-formyl-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylate (152A4-00). In conclusion, the method provided by the invention is more beneficial to safe, simple and cost-efficientindustrial scale preparation of the febuxostat intermediate with higher purity.
Owner:内蒙古京东药业有限公司 +1
Method of preparing adapalene
InactiveCN101033190AHigh costThorough responseOrganic compound preparationCarboxylic compound preparationSolventBromine
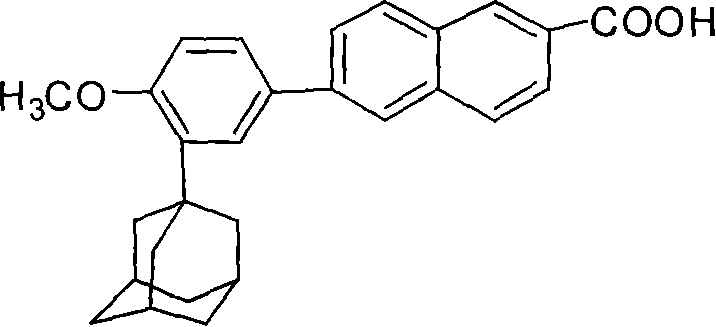
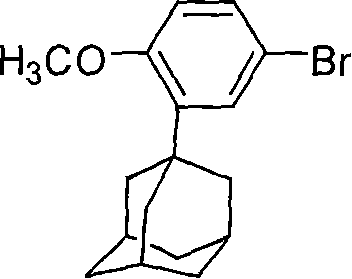
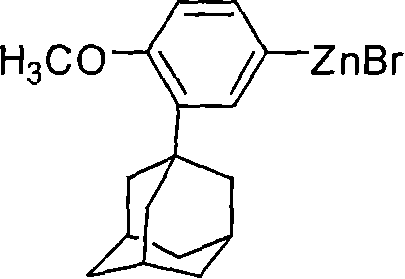
This invention relates to a new method for synthesizing Adapalene 6-[3-(1-King Kong alkyl)-2-naphthoic acid, in which, 2-(1-King Kong alkyl)-4-bromophenyl methyl ether and Zn generate organic Zn reagent in aether-like or non-polarity solvent, which is reacted with 6-bromine-2-naphthoic acid ethyl and analyzed to get the Adapalene.
Owner:北京精华耀邦医药科技有限公司
Method for analyzing genotoxic impurities in moxifloxacin hydrochloride starting material
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to a quantitative analysis method for potential genotoxic impurities in moxifloxacin hydrochloride starting raw material 1-cyclopropyl-6, 7-difluoro-1, 4-dihydro-8-methoxy-4-oxo-3-quinoline carboxylic acid ethyl ester, and the structure types of the potential genotoxic impurities comprise acyl halidesand unsaturated ketones. According to the invention, absolute ethyl alcohol and 2, 4-dichlorophenol are adopted; the method comprises the following steps: carrying out a derivatization reaction on 2,4, 5-trifluoro-3-methoxybenzoyl chloride to generate ethyl 2, 4, 5-trifluoro-3-methoxybenzoate, and carrying out separation and mass spectrometry detection by adopting (5% phenyl)-methyl polysiloxaneas a fixed phase derivative ethyl 2, 4, 5-trifluoro-3-methoxybenzoate and an impurity ethyl 3-(N, N-dimethylamino) acrylate. The method not only can realize effective separation of potential genotoxic impurities in the initial raw material, but also can quantitatively detect the genotoxic impurities, is simple, convenient and rapid to operate, strong in specificity, high in sensitivity, accurateand reliable, and has important significance for quality control and medication safety of moxifloxacin hydrochloride raw materials and preparations.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +1
Synthesis method of ethyl tetrazole-5-carboxylate
The invention relates to a method for synthesizing ethyl tetrazole-5-carboxylate, which belongs to the field of organic synthesis and comprises the following steps of: (1) heating and refluxing by taking ethyl cyanoformate and sodium azide as raw materials and a mixed solution of methylbenzene and water as a solvent to generate ethyl tetrazole-5-carboxylate sodium salt; (2) heating and distillingthe reflux liquid obtained in the step (1) to remove methylbenzene; (3) neutralizing the distillate obtained in the step (2) with an acidic solution, and conducting separating to obtain a crude product of ethyl tetrazole-5-carboxylate; and (4) recrystallizing the crude product of ethyl tetrazole-5-carboxylate through a mixed solvent of low-carbon hydrocarbon and water, and conducting separating toobtain ethyl tetrazole-5-carboxylate. The synthesis method disclosed by the invention is simple to operate and high in yield, and the purity of the prepared ethyl tetrazole-5-carboxylate is high.
Owner:河北凯诺中星科技有限公司 +1
Preparation method of diclazuril impurity A
InactiveCN111517991AShort synthetic routeStarting materials are readily availableCarbamic acid derivatives preparationOrganic compound preparationAcyl groupFormic acid ethyl ester
The invention discloses a preparation method of a diclazuril impurity A. The diclazuril impurity A is obtained through the procedures of condensation reaction of a diclazuril intermediate (3-butyloxycarbonylamino-3-oxopropionyl)-butyl carbamate or (3-ethoxycarbonylamino-3-oxopropionyl)-ethyl carbamate (compound I) used as a starting material with sodium nitrite in a polar solvent and under acid catalysis, decoloration, crystallization and drying. The synthesis route is short, the starting material is easy to obtain, reaction conditions are mild, operation is easy and convenient, the impurity can be obtained without column chromatography, the purity of the prepared impurity can reach 99.5% or above, and the impurity can serve as a reference substance to be used for quality research.
Owner:CHANGZHOU YABANG QH PHARMACHEM +2
Process for the preparation of umeclidinium bromide
ActiveUS10759801B2Improve chemical reaction efficiencySave resourcesPowder deliveryOrganic active ingredientsOrganic baseEthyl group
The present invention discloses processes comprising a) reacting ethyl isonipecotate with 1-bromo-2-chloroethane in the presence of an organic base in a solvent to form ethyl 1-(2-chloroethyl)piperidine-4-carboxylate (II) or a salt thereof. Process step a) may be included in a process for preparing umeclidinium bromide that comprises further process steps: b) reacting ethyl 1-(2-chloroethyl)piperidine-4-carboxylate (II) or a salt thereof with lithium diisopropylamide in a solvent to form ethyl 1-azabicyclo[2.2.2]octane-4-carboxylate (III); c) reacting ethyl 1-azabicyclo[2.2.2]octane-4-carboxylate (III) with phenyl lithium in a solvent to form 1-azabicyclo[2.2.2]oct-4-yl(diphenyl)methanol (IV); and d) reacting 1-azabicyclo[2.2.2]oct-4-yl(diphenyl)methanol (IV) with ((2-bromoethoxy)methyl) benzene in a solvent to form 4-[hydroxyl(diphenyl)methyl]-1-[2-(phenylmethyl)oxy]ethyl]-1-azoniabicyclo[2.2.2]octane bromide (I), umeclidinium bromide. A process comprising d) reacting 1-azabicyclo[2.2.2]oct-4-yl(diphenyl)methanol with ((2-bromoethoxy)methyl) benzene in a solvent to form 4-[hydroxyl(diphenyl)methyl]-1-[2-(phenylmethyl)oxy]ethyl]-1-azoniabicyclo[2.2.2]octane bromide (I), umeclidinium bromide, wherein the solvent is selected from cyclic ethers such as tetrahydrofuran, aromatic solvents, such as toluene, ketones such as acetone and protic solvents such as water or combinations thereof, optionally wherein the solvent is water is also disclosed. Umeclidinium bromide obtainable from the disclosed processes, ethyl 1-(2-chloroethyl)piperidine-4-carboxylate (II) and pharmaceutical compositions are also disclosed.
Owner:HOVIONE SCIENTIA
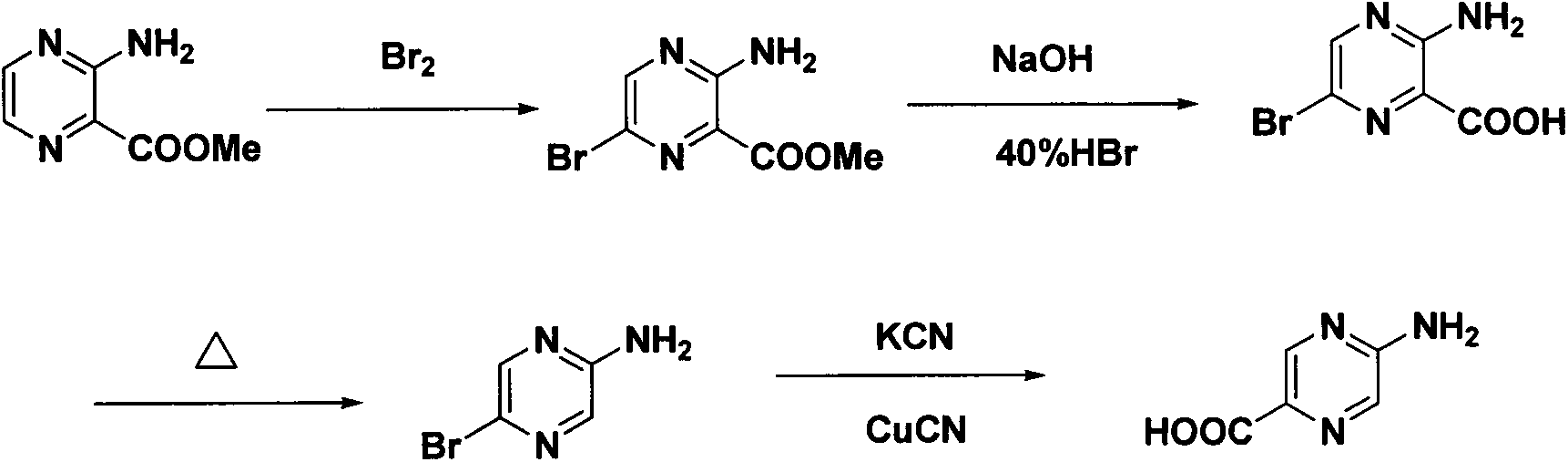
Synthesis method of 2-aminopyrazinyl-5-formic acid
The invention discloses a novel synthesis method of 2-aminopyrazinyl-5-formic acid, which comprises the following steps: carrying out hydrazinolysis reaction on accessible 2,5-ethyl diformate pyrazine to hydrazinolyze the monoester, reacting with a sodium nitrate solution, refluxing in toluene and tert-butyl alcohol to obtain a key intermediate 2-ethyl formate-5-Boc-amino-pyrazine, and refluxing in hydrochloric acid to obtain the 2-aminopyrazinyl-5-formic acid. The method has the advantages of mild and simple reaction conditions, low cost and high yield, is simple to operate and suitable for industrial production, and has great economic significance.
Owner:苏州科捷生物医药有限公司
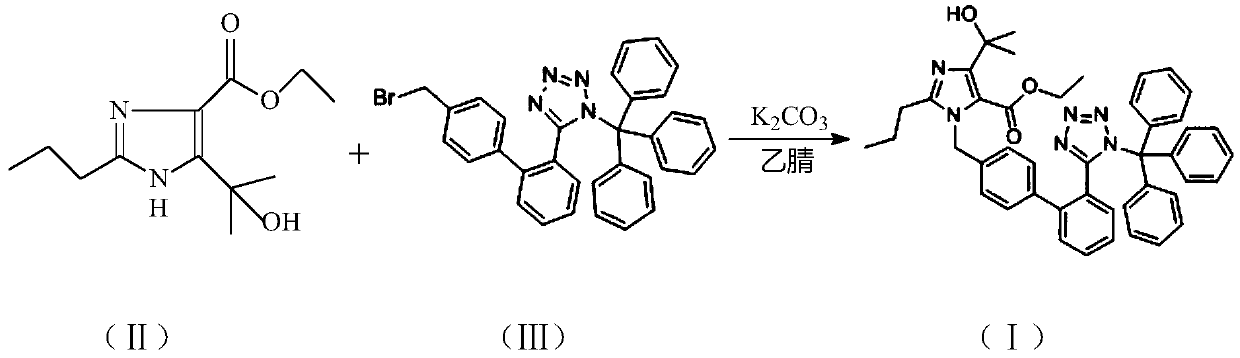
Preparation and purification method of olmesartan medoxomil key intermediate
The invention relates to a preparation and purification method of a key intermediate 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2' -(triphenylmethyl-1H-tetrazole-5-yl) (1, 1'-biphenyl)-4-yl] methyl}-1H-imidazole-5-ethyl formate (I) for preparing a chemical drug olmesartan medoxomil for treating hypertension. The invention provides a preparation and purification method for generating a compound (I) by reacting 4-(1-hydroxy-1-methylethyl)-2-propyl-1H-imidazole-5-carboxylic acid ethyl ester (II) with N-(triphenylmethyl)-5-(4'-bromomethylbiphenyl-2-yl) tetrazole (III) in the presence of an organic solvent and an acid-binding agent. According to the preparation and purification method of the compound (I), the impurity content is effectively reduced, and the quality of a subsequent target productis improved. The method is stable in process, high in yield, good in quality, simple to operate, less in three wastes, low in production cost and suitable for industrial production, and the recoveredsolvent can be continuously used.
Owner:SHANDONG XINHUA PHARMA CO LTD
Continuous hydrogenation method and application of ethyl pyrazine-2-carboxylate
ActiveCN111559983ARaise the degree of adequate responseShort reaction timeOrganic chemistryTemperature controlPyrazine
Owner:ASYMCHEM LIFE SCI TIANJIN
Method for preparing 1-ferrocenyl-3-aryl-3-(ethyl dicarboxylate methine)-acetone
The invention discloses a method for preparing 1-ferrocenyl-3-aryl-3-(ethyl dicarboxylate methine)-acetone, and the method comprises the following steps: adding choline chloride and urea into a dry three-necked flask, and stirring to a transparent solution at 80 DEG C to obtain a deep eutectic solvent; cooling to room temperature, adding ferrocenyl chalcone and diethyl malonate, slowly heating, refluxing for reacting, and performing TLC monitoring until the reaction is finished; cooling the reaction mixture to room temperature, adding a small amount of water, immediately precipitating solids,performing suction filtering, washing the filter cake with water, and drying to obtain the 1-ferrocenyl-3-aryl-(ethyl dicarboxylate methine)-acetone with a yield of over 81%. The filtrate is concentrated and recovered to obtain the deep eutectic solvent, which can be reused. The invention provides a simple, convenient, green and environment-friendly method for the synthesis of the compound, meanwhile, the catalyst can be recycled, and the reaction cost is reduced.
Owner:SHAANXI UNIV OF SCI & TECH
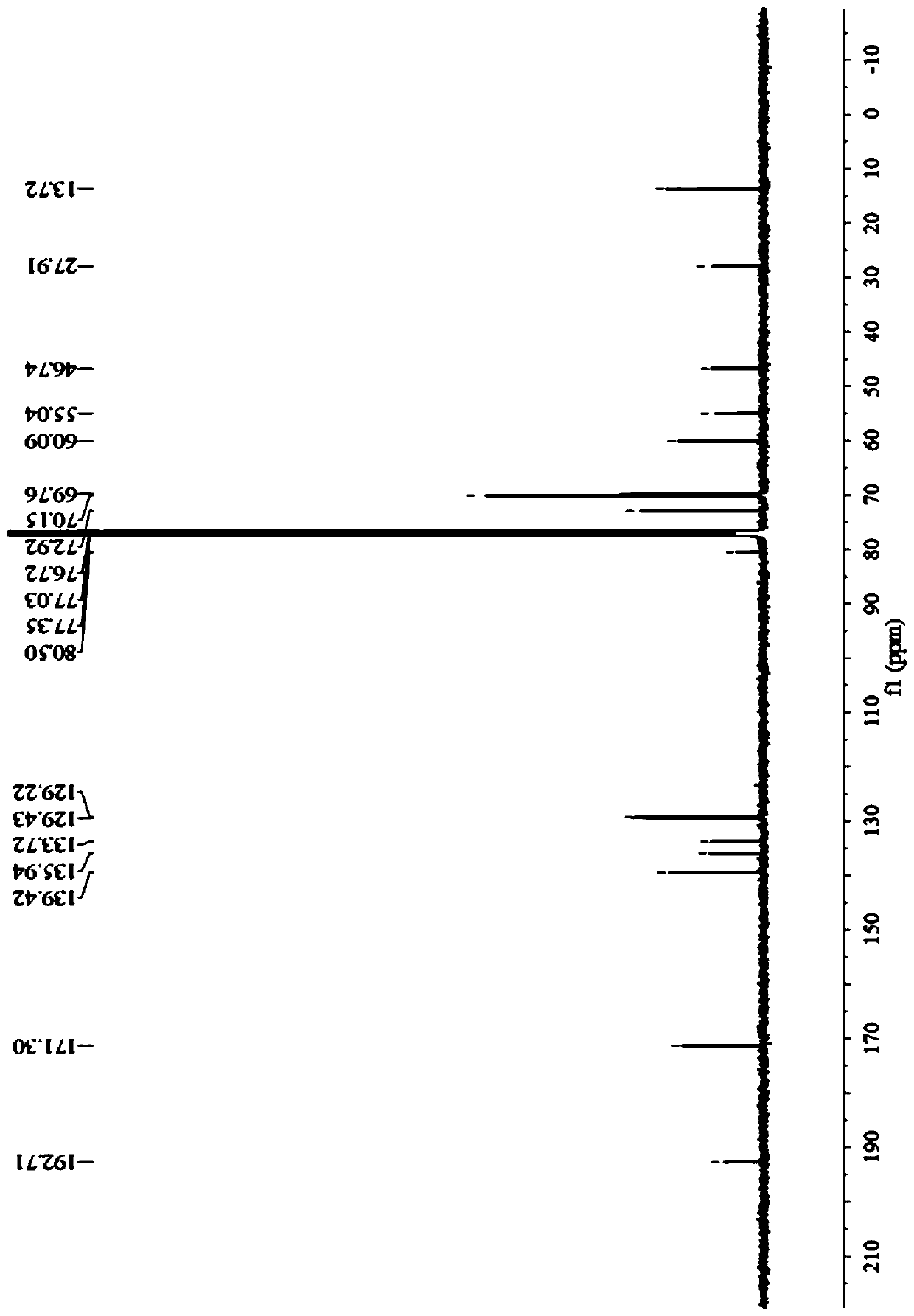
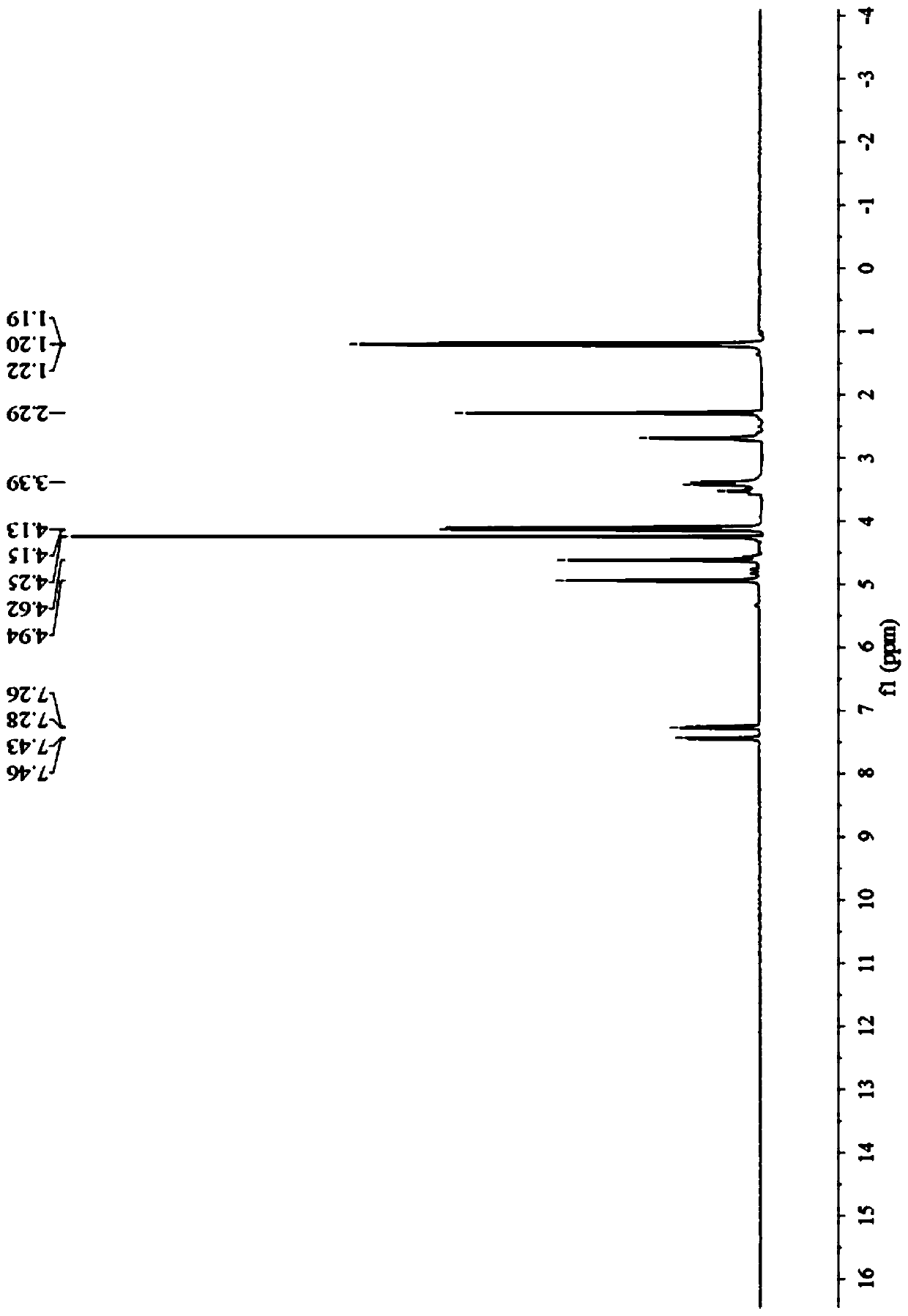
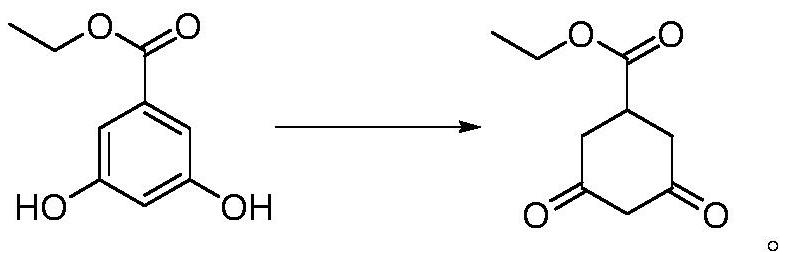
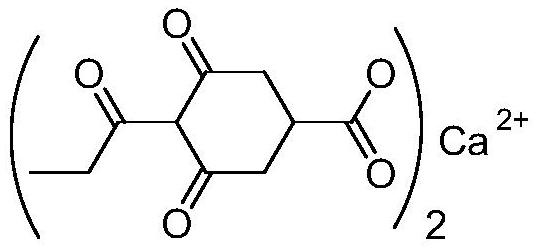
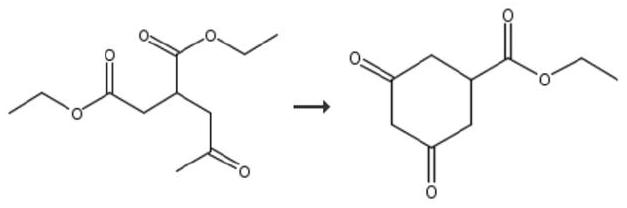
Preparation method of prohexadione calcium intermediate
PendingCN114805067AReduce usageEasy to operateOrganic compound preparationCarboxylic acid esters preparationEthyl hydroxybenzoateBenzoic acid
The invention discloses a preparation method of a prohexadione calcium intermediate. The method comprises the following steps: dissolving 3, 5-ethyl dihydroxybenzoate in a solvent, adding formic acid and a catalyst, heating to 100-150 DEG C for reaction, cooling to 20-30 DEG C after the reaction is finished, filtering to recover the catalyst, and carrying out reduced pressure evaporation on filtrate to recover unreacted formic acid and the solvent, thereby obtaining the 3, 5-dioxocyclohexyl ethyl formate. According to the process, acetone is not used, a large amount of waste salt is not generated, and the process is environment-friendly, high in yield and purity and suitable for large-scale industrial production.
Owner:ZHEJIANG UNIVERSITY OF SCIENCE AND TECHNOLOGY
A kind of synthetic method of the amine compound containing sulfur nitrogen heterocycle of medicine intermediate
ActiveCN108084111BFew synthetic stepsImprove creativityOrganic chemistryWater methanolTetrahydrofuran
The invention relates to a method for synthesizing a pharmaceutical intermediate sulfur-containing nitrogen heterocyclic amine compound. The method comprises that ethyl 4-methyl-3-oxo-pentanoate, dichloromethane and sulfonyl chloride as raw materials undergo a reaction, the reaction product is subjected to pH adjustment until pH of 7, the treated reaction product is extracted so that ethyl 2-chloro-4-methyl-3-oxopentanoate is obtained, the ethyl 2-chloro-4-methyl-3-oxopentanoate and thiourea undergo a reaction in the presence of ethanol as a solvent to produce ethyl 2-amino-4-isopropylthiazole-5-formate, isoamyl nitrite is added into ethyl 2-amino-4-isopropylthiazole-5-formate in the presence of tetrahydrofuran as a solvent to obtain 4-isopropylthiazole-5-formate, a NaOH solution is addedinto the 4-isopropylthiazole-5-formate in the presence of anhydrous methanol as a solvent so that 4-isopropylthiazole-5-formic acid is obtained, the 4-isopropylthiazole-5-formic acid and azophosphoryldiphenyl ester undergo a reaction in the presence of tert-butyl alcohol as a solvent and triethylamine, the reaction product is extracted, the extract is washed so that 5-N-BOC-4-isopropylthiazole isobtained, the 5-N-BOC-4-isopropylthiazole and HCl undergo a reaction in the presence of anhydrous methanol as a solvent to produce 4-isopropyl-5-aminothiazole. The method has the advantages of clearprocesses, less waste, high yield, raw material saving and operation easiness.
Owner:烟台宁远药业有限公司
A kind of preparation method of pranlukast
The invention belongs to the field of medicines and especially relates to a preparation method for pranlukast. The method comprises the following steps: A) adding 4-bromobenzoic acid and thionyl chloride into a solvent and reacting, thereby acquiring 4-bromo-benzoyl chloride; B) adding the 4-bromo-benzoyl chloride acquired in the step A), alkali and 3-amino-2-ethyl iodobenzoate into the solvent and reacting, thereby acquiring 2-iodine-3-(4-bromobenzamide) ethyl benzoate; C) adding the 2-iodine-3-(4-bromobenzamide) ethyl benzoate acquired in the step B), alkali and 1-(1H-tetrazole-5-group) aceton into the solvent and reacting, thereby acquiring 4-bromine-N-(4-oxo-2-(1H-tetrazole-5-group)-4H-benzopyrone-8-group) benzamide; D) adding the 4-bromine-N-(4-oxo-2-(1H-tetrazole-5-group)-4H-benzopyrone-8-group) benzamide acquired in the step C), catalyst and alkali into the solvent and reacting, thereby acquiring the product. The method provided by the invention is simple and practicable in process, low in production cost and high in yield.
Owner:烟台万润药业有限公司
Synthetic method of 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid
InactiveCN106831782AReaction raw materials are readily availableEasy to operateOrganic chemistryN dimethylformamidePyridazine
The invention relates to a synthetic method of 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid. The synthetic method comprises the steps of reacting N,N-dimethylformamide dimethyl acetal and 3-amino-6-chloropyridazine at the temperature of 40 to 120 DEG C to obtain an intermediate; under an alkali action, reacting at the temperature of 65 to 140 DEG C, and carrying out rotary evaporation and concentration to obtain a 6-chloroimidazo [1,2-b]pyridazine-3-formic acid ethyl ester crude product; recrystallizing the crude product to obtain a pure product; under an alkali action, carrying out hydrolysis reaction in a certain solvent, finishing reaction, neutralizing through hydrochloric acid, carrying out suction filtration, and washing drying to obtain a 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid pure product. The synthetic method provided by the invention is adopted for preparing the 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid, so that the reaction raw materials can be obtained easily, the method is easy to operate and control and simple in aftertreatment, and the product has stable quality and high purity.
Owner:陕西友帮生物医药科技有限公司
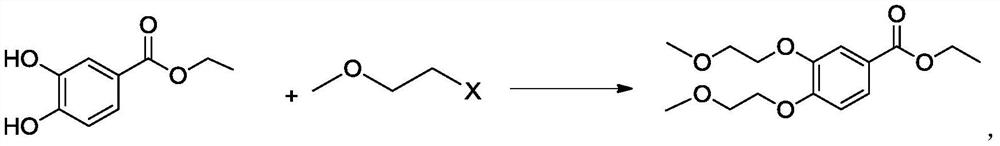
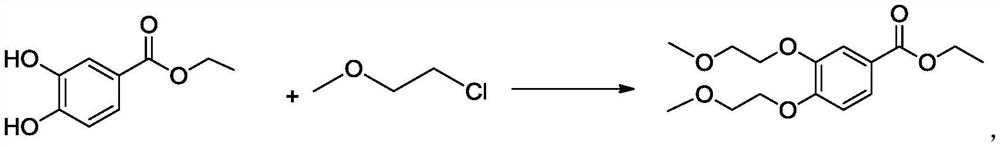
A kind of preparation method of 3,4-two (2-methoxyethoxy) ethyl benzoate
ActiveCN106892821BThe preparation method is simple and environmentally friendlySuitable for industrial scale productionOrganic compound preparationCarboxylic acid esters preparationBenzoic acidOrganic solvent
The invention belongs to the field of drug synthesis, and relates to a preparation method of an anticancer drug intermediate, in particular to a preparation method of ethyl 3,4-bis(2-methoxyethoxy)benzoate. This preparation method has research reports in many documents or patents, but all need the participation of organic solvents such as acetonitrile, acetone or DMF. The present invention does not need to use the solvent in the existing documents or patents, but can obtain products with high yield and purity, is a green and environment-friendly preparation method, and is suitable for industrial production.
Owner:ZHEJIANG JIUZHOU PHARM CO LTD
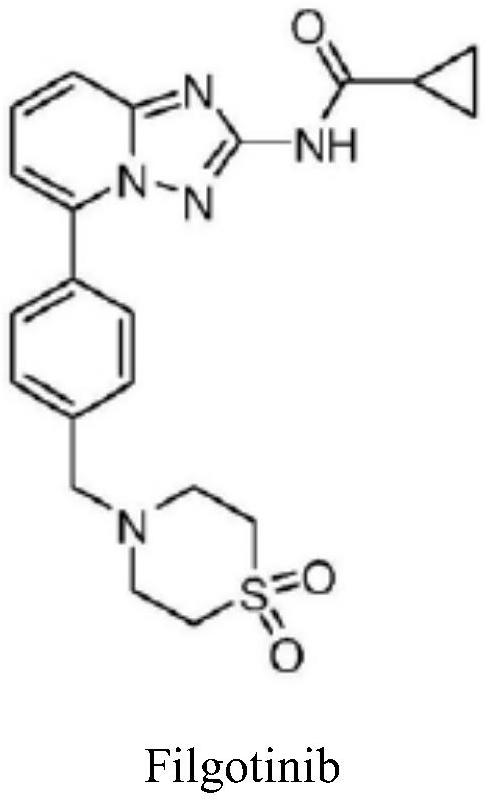
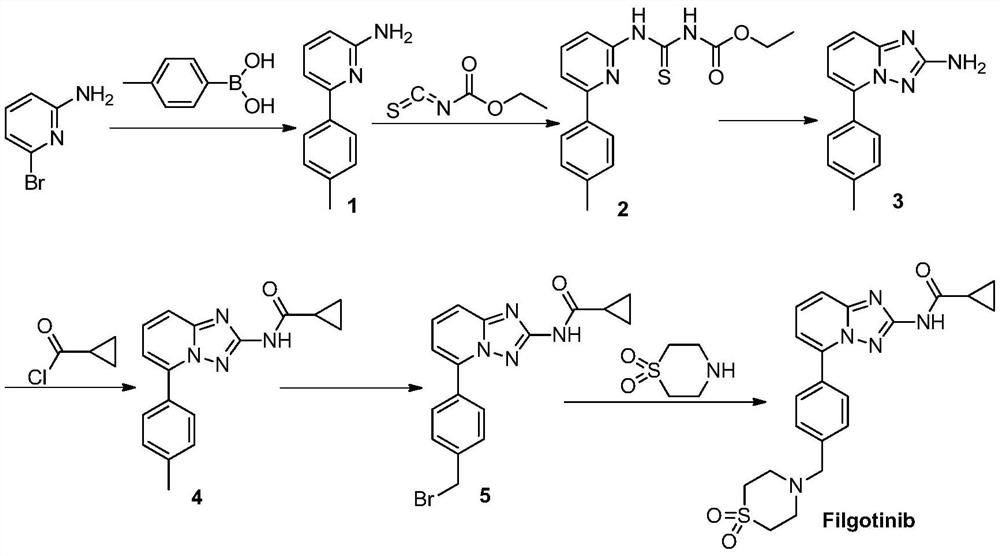
A kind of synthetic method of jak1 inhibitor filgotinib
The invention provides a preparation method of Filgotinib, comprising the following steps: (1) reacting 2-amino-6-bromopyridine with p-toluene derivatives to prepare compound 1; (2) compound 1 and isothiocyanate formic acid Ethyl reaction to prepare compound 2; (3) compound 2 reacted with hydroxylamine hydrochloride and N,N-diisopropylethylamine to prepare compound 3; (4) compound 3 reacted with cyclopropanoyl chloride to obtain compound 4; ( 5) Compound 4, N-bromosuccinimide was reacted with azobisisobutyronitrile to obtain compound 5; (6) Compound 5 was reacted with thiomorpholine-1,1-dioxide to obtain Filgotinib. The route for synthesizing Filgotinib in the present invention is coupling first and then ring closing, the raw materials are cheap, the reaction operation is simple, the product is easy to purify, and the yield is high, which is suitable for commercial scale production.
Owner:四川伊诺达博医药科技有限公司
Analysis method of genotoxic impurity in moxifloxacin hydrochloride starting material
The invention belongs to the technical field of drug analysis, in particular to a moxifloxacin hydrochloride starting material 1-cyclopropyl-6,7-difluoro-1,4-dihydro-8-methoxy-4-oxo- Quantitative analysis method for potential genotoxic impurities in ethyl 3‑quinolinecarboxylate. The structure types of potential genotoxic impurities include acid halides and unsaturated ketones. The present invention adopts absolute ethanol and 2,4,5-trifluoro-3-methoxybenzoyl chloride to carry out derivatization reaction to generate 2,4,5-trifluoro-3-methoxybenzoic acid ethyl ester, adopts ( 5% phenyl)-methyl polysiloxane is a fixed relative derivative 2,4,5-trifluoro-3-methoxy ethyl benzoate and impurity 3-(N,N-dimethylamino)acrylic acid Ethyl esters were separated and detected by mass spectrometry. The method can not only realize the effective separation of potential genotoxic impurities in the starting raw materials, but also can quantitatively detect the genotoxic impurities. The operation is simple and fast, with strong specificity, high sensitivity, accuracy and reliability. It is of great significance to the quality control and drug safety of preparations.
Owner:SHANDONG INST FOR FOOD & DRUG CONTROL +1
A kind of preparation method of ethyl benzoate
ActiveCN109160880BHigh selectivityImprove conversion rateOrganic compound preparationCarboxylic acid esters preparationPtru catalystPhysical chemistry
The invention discloses a preparation method of ethyl benzoate, comprising the following steps: putting sodium benzoate, toluene, and a phase transfer catalyst into a pressure kettle, replacing the air in the kettle with nitrogen, and feeding ethyl chloride at a certain temperature and pressure. After the reaction, the catalyst and the sodium chloride produced by the reaction are filtered off, the solvent toluene is recovered, and the finished product is obtained by rectification under reduced pressure. Compared with the current esterification process, the present invention has the advantages of high product yield, no serious corrosion of equipment, zero discharge of "waste water", and the like.
Owner:HUAIAN WAN BANG SPICE IND CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







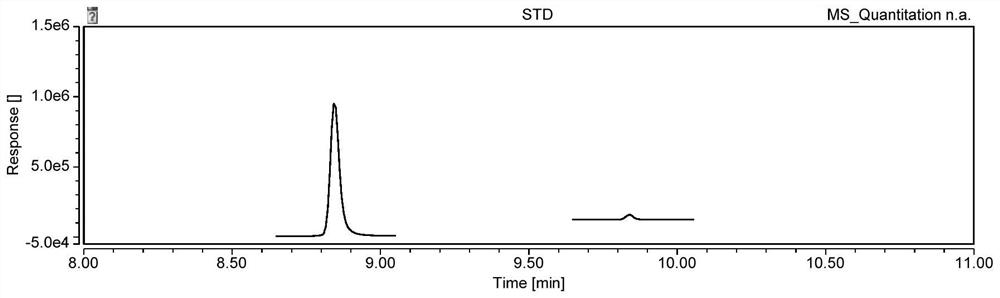
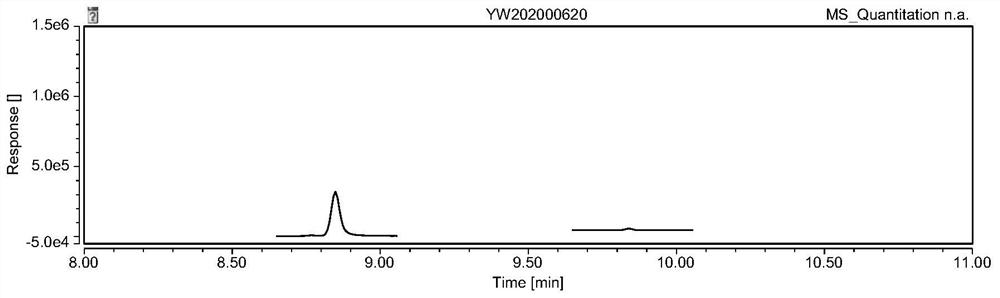
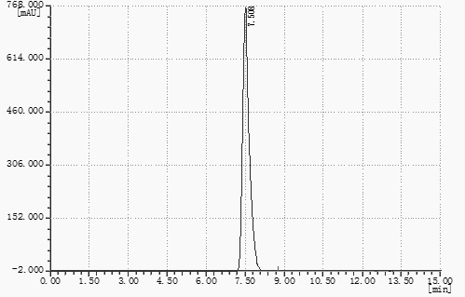
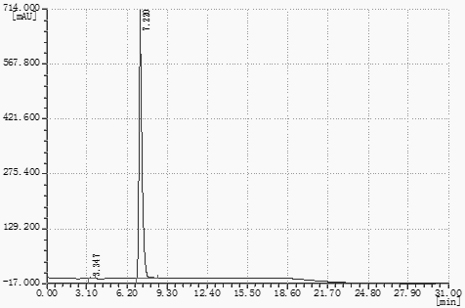
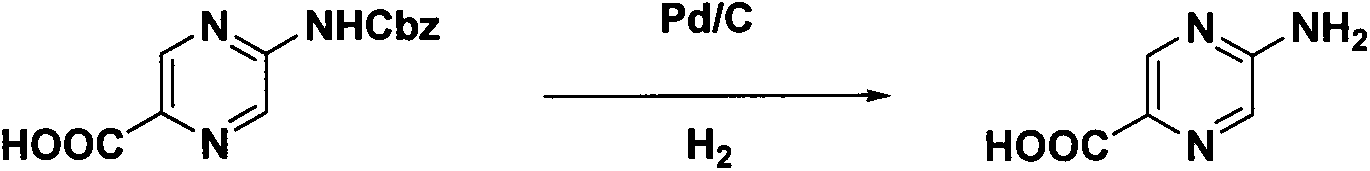


![Synthetic method of 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid Synthetic method of 6-chloroimidazo [1, 2-b]pyridazine-3-formic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/43b77bae-9acd-48ff-9770-d71065712034/DEST_PATH_IMAGE001.png)