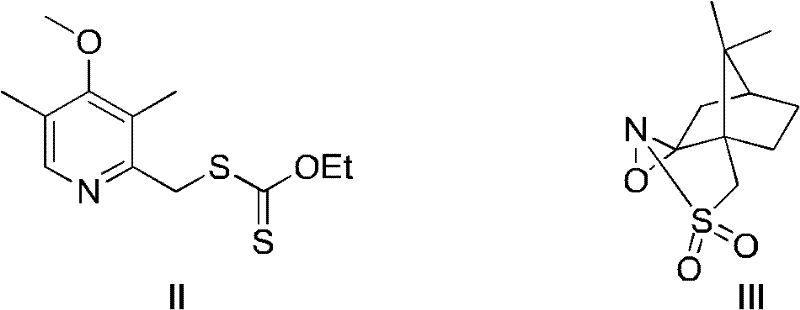
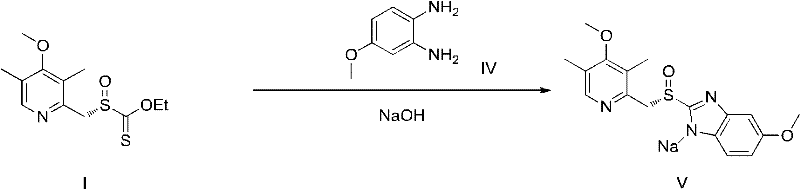
Novel chiral sulfoxide compound and method for preparing esomeprazole by using novel chiral sulfoxide compound
A compound, sodium hydroxide technology, applied in the direction of organic chemistry and the like, can solve the problems of low yield, expensive reagents, expensive synthetic raw materials and chiral resolving agents, etc., and achieves the effects of mild reaction conditions and short synthetic process steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Preparation of Intermediate II ((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)-thioxanthogenate ethyl ester
[0024]
[0025] At room temperature, 2.22kg 2-(chloromethyl)-4-methoxy-3,5-lutidine hydrochloride was dissolved in 150L ethanol, 1.38kg potassium carbonate was added in batches, and after stirring for 10 minutes , 1.92 kg potassium xanthate was added, and the reaction solution was stirred at room temperature for about 3 hours (detected by TLC). The resulting potassium chloride solid was filtered, washed with ethanol, and the filtrate was concentrated. Add 80L of water to the residue, stir, filter the obtained solid, and recrystallize with ethyl acetate-petroleum ether to obtain pure ((4-methoxy-3,5-dimethylpyridin-2-yl) (Methyl)-thioxanthogenate (2.66 kg, yield 98%, pale yellow solid).
[0026] 1 H-NMR(300MHz, CDCl 3 ): δ 8.18 (s, 1H), 4.67 (q, 2H), 4.54 (s, 2H), 3.76 (s, 3H), 2.30 (s, 3H), 2.24 (s, 3H), 1.43 (t, 3H). EIMS m / z272.1([M+H] + ).
Embodiment 2
[0027] Example 2 Preparation of Compound I (S)-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl) thioformate
[0028]
[0029] Dissolve 2.5kg of Intermediate II in 200L of isopropanol at 70°C, add 2.1kg of DBU and 2.3kg (11eq.) of self-made oxidant III in sequence, stir the reaction solution for 12 hours, filter the white solid produced, and use isopropyl alcohol for the solid. Wash with propanol, spin the filtrate to dryness, dissolve the residue in water, filter the white solid produced again, add acetic acid to the filtrate to adjust the pH to 7.2, then extract with ethyl acetate, dry the organic phase, and spin-evaporate to remove the solvent. The white solid is the crude product. It is recrystallized from ethyl acetate-petroleum ether to obtain pure (S)-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinic acid Acyl) ethyl methanesulfonate (2.25 kg, yield 85%, white solid).
[0030] 1 H-NMR(300MHz, CDCl 3 ): δ 8.25 (s, 1H), 4.15 (s, 2H), 3.80 (s, 3H), 3.60 (q, 2H), 2.33 ...
Embodiment 3
[0031] Example 3 Preparation of Compound I (S)-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl) thioformate
[0032]
[0033] Dissolve 500g of Intermediate II in 40L of isopropanol at 10°C, add 420g of DBU and 380g (0.9eq.) of self-made oxidant III in sequence, stir the reaction solution for 12 hours, filter the white solid produced, and use isopropanol for the solid Wash, spin the filtrate to dryness, dissolve the residue in water, filter the white solid produced again, add acetic acid to the filtrate to adjust the pH to 7.6, then extract with ethyl acetate, dry the organic phase, and remove the solvent by rotary evaporation to obtain an off-white solid It is a crude product and recrystallized with ethyl acetate-petroleum ether to obtain pure (S)-(((4-methoxy-3,5-dimethylpyridin-2-yl)methyl)sulfinyl ) Ethyl thioformate (375.9 g, yield 71%, white solid). The NMR and MS data are consistent with Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com