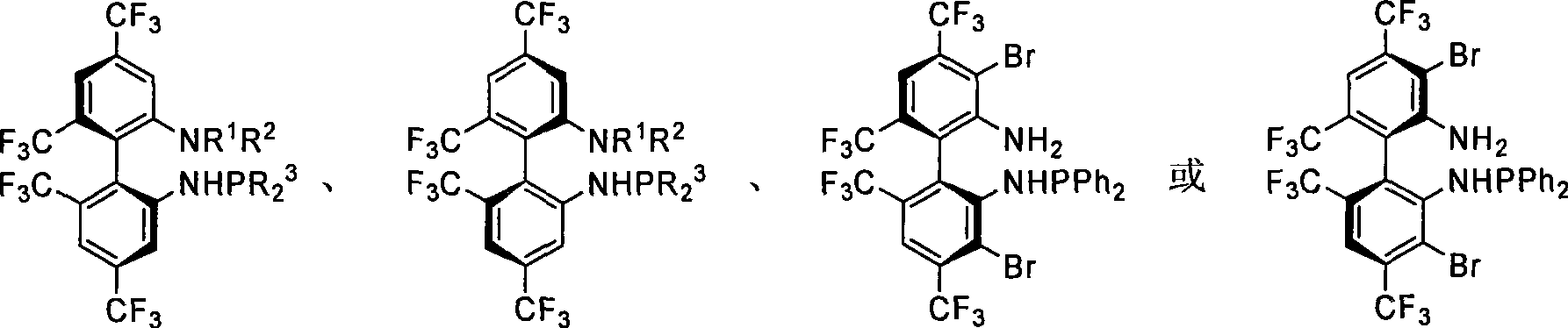
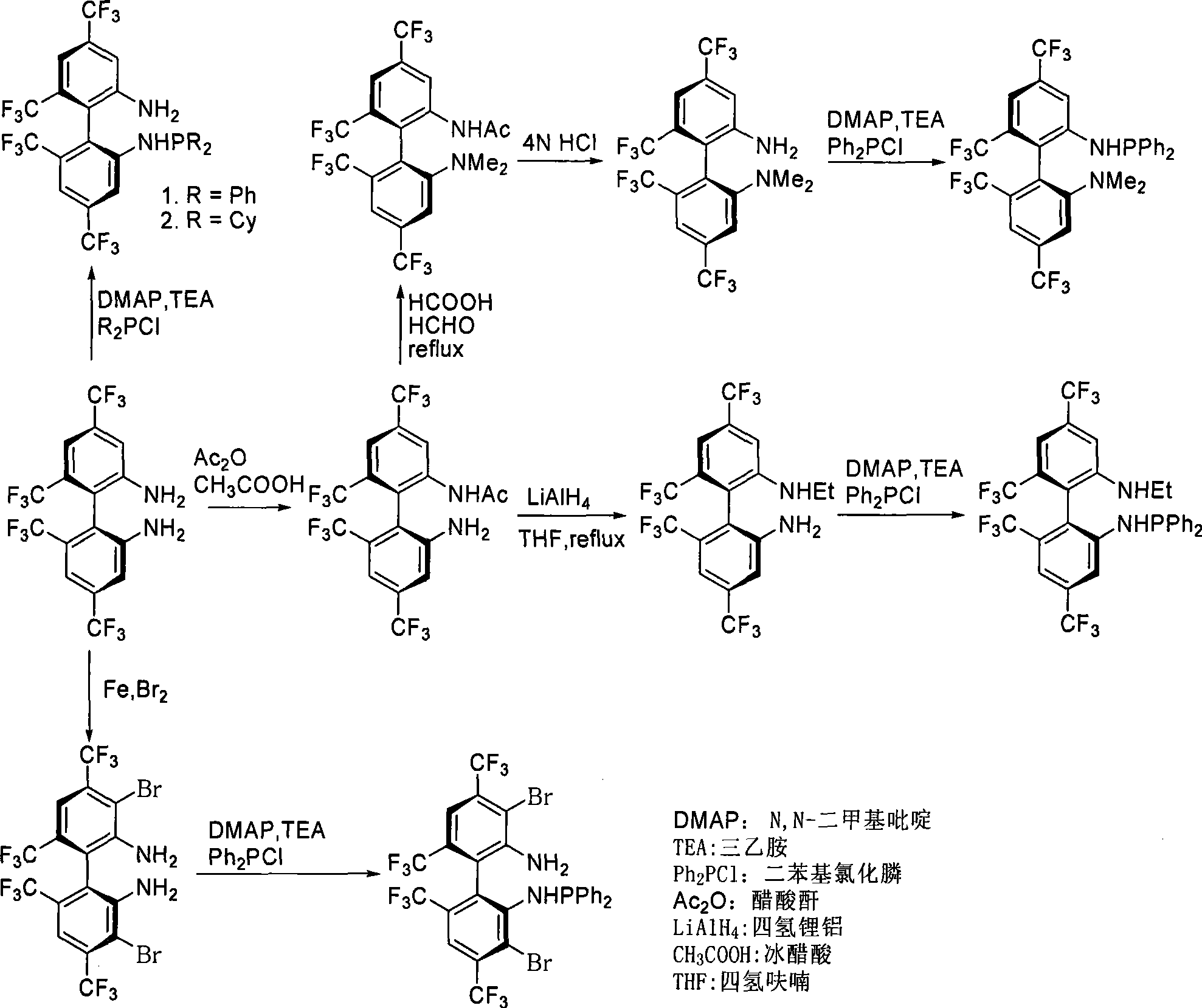
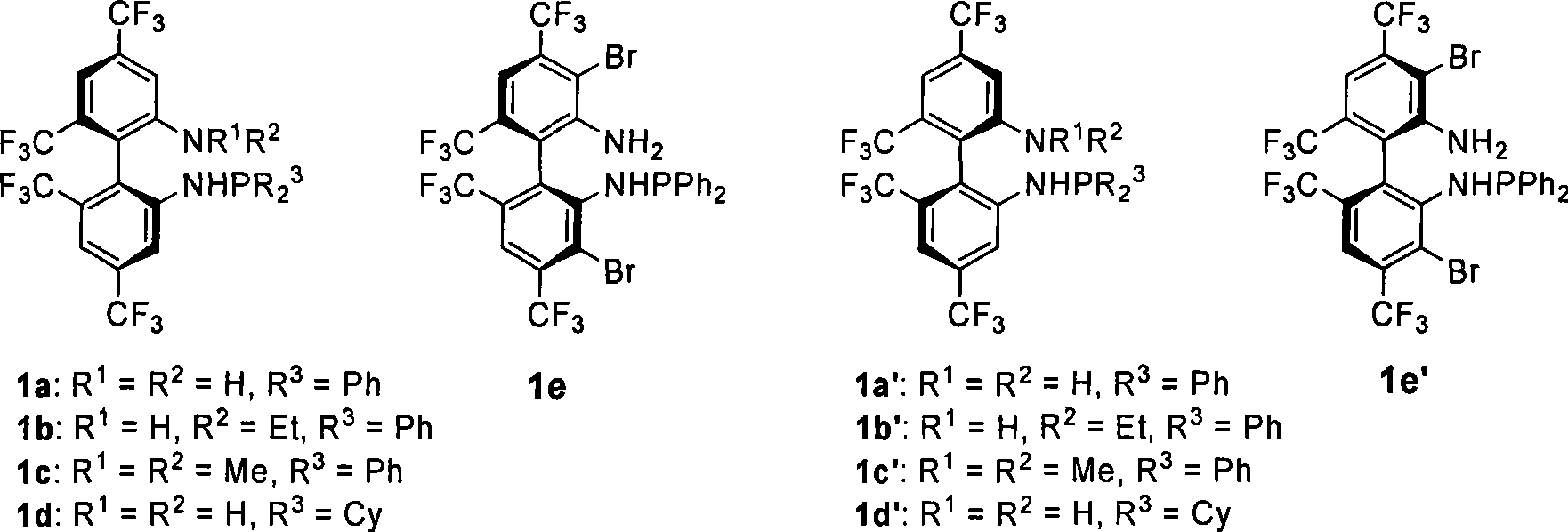
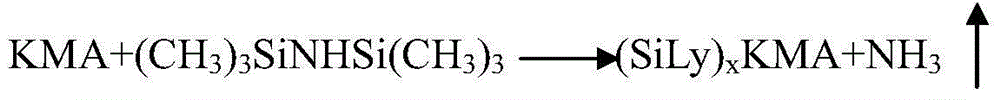Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
244 results about "Dimethpyrindene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Combination of a purine-based cdk inhibitor with a tyrosine kinase inhibitor and use thereof in the treatment of proliferative disorders
InactiveUS20100143350A1Low toxicityGood effectBiocideAntibody ingredientsErbBTyrosine-kinase inhibitor
The present invention relates to combination comprising (i) an ErbB inhibitor; and (ii) a CDK inhibitor, or a pharmaceutically acceptable salt thereof, selected from: (a) roscovitine; (b) 3-{9-isopropyl-6-[(pyridin-3-ylmethyl)-amino]-9H-purin-2-ylamino}-2-methyl-pentan-2-ol; (c) 3-{9-isopropyl-6-[(pyridin-3-ylmethyl)-amino]-9H-purin-2-ylamino}-pentan-2-ol; and (d) (2R,3S-3-(6-((4,6-dimethylpyridin-3-ylmethylamino)-9-isopropyl-9H-purin-2-ylamino)pentan-2-ol.Further aspects of the invention relate to pharmaceutical products and pharmaceutical compositions comprising combinations according to the invention, and methods of treatment using the same.
Owner:CYCLACEL
Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides
ActiveUS20050215570A1Easy to optimizeQuick responseBiocideOrganic active ingredientsArylSulfonyl chloride
Owner:CORTEVA AGRISCIENCE LLC
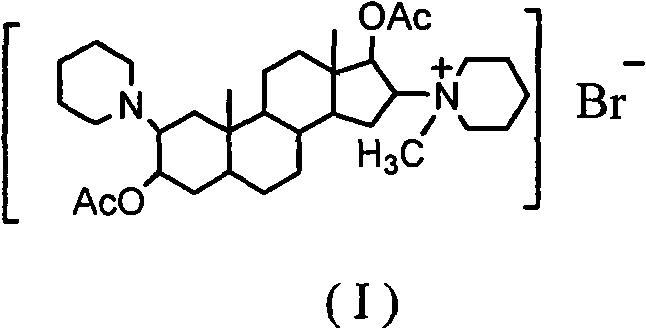
Synthesis process of vecuronium bromide
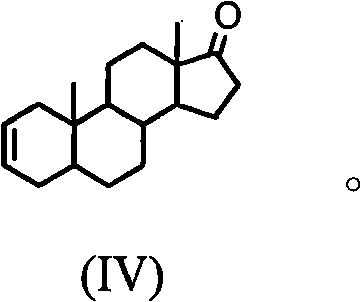
The invention discloses a synthesis process of vecuronium bromide. The synthesis process comprises the following steps: generating epiandrosterone sulfonyl ester (III) by carrying out esterification reaction between epiandrosterone (II) and paratoluensulfonylchloride; generating 5Alpha-androst-2-alkene-17-ketone (IV) by carrying out elimination and dehydration reaction between the (III) and 2,6-lutidines; generating 17-acetoxyl-5Alpha-androstane-2,16-diene (V) by carrying out enolization and esterification reaction between the (IV) and isopropenyl acetate; generating (2Alpha, 3Alpha, 16Alpha,17Alpha)-diepoxy-17Beta-acetyl-5Alpha-androstane (VI) by epoxy reaction of the (V) under the effect of hydrogen peroxide; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha-hydroxyl-17-ketone (VII) by ring-opening and addition reaction of the (VI) under the effect of hexahydropyridine; generating 2Beta, 16Beta-di(1-piperidyl)-5Alpha-androstane-3Alpha,17Beta-diol (VIII) by the (VII)under the reduction of potassium borohydride; generating 2Beta, 16Beta-di(1-piperidyl)-3Alpha, 17Beta- acetoxyl-5Alpha-androstane (IX) by carrying out esterification reaction of the (VIII) under the acetylation of acetic anhydride; and generating vecuronium bromide (I) by carrying out quaternary ammonium salt reaction between the (IX) and bromomethane. The invention has the advantages of low cost,less pollution and high yield.
Owner:XUZHOU NORMAL UNIVERSITY
Methyl pyridinium with organic two-photon absorption materia, and preparation method and application thereof
InactiveCN102702090APromote absorptionGood water solubilityOrganic chemistryMaterial analysis by observing effect on chemical indicatorSolubilitySolvatochromism
The invention relates to the field of organic nonlinear optical materials and relates to methyl pyridinium with an organic two-photon absorption material, and a preparation method and the application of the methyl pyridinium. The structural formula of the product is described in structural formula (I); the preparation method comprises the steps of taking 2,6-dimethyl pyridine as a raw material and finally obtaining a compound (I) by conducting oxidizing reaction, esterification reaction, methylolation reaction, halogenation reaction, nucleophilic addition reaction, Wittig reaction and the final methylation reaction. The raw materials are easy to access, and the synthesis steps are simple. The compound (I) has excellent two-photon absorption performance and good water solubility and is an ideal biological fluorescence probe; an obvious solvatochromism phenomenon easily occurs, the compound can be used for detecting water content in a common organic solvent, can also be used as a fluorescent probe for detecting the PH value in a solution system, has a series of advantages of high sensibility, quick response, identification by naked eyes and the like, and has an excellent application prospect.
Owner:SHANGHAI NORMAL UNIVERSITY
Synthesis method for dehydroepiandrosterone
InactiveCN102212099AOvercoming reactivityOvercoming processingSteroidsBeckmann rearrangementSodium acetate
The invention belongs to a novel synthesis method for dehydroepiandrosterone, which comprises the following steps: oximation of 16-dehydropregnenolone acetate, Beckmann rearrangement, hydrolysis and refining to obtain a product. The synthesis method is characterized by carrying out oximation of ketone by using sodium acetate as a base and water and ethanol as solvent, carrying out Beckmann rearrangement, hydrolysis and one-pot refining reaction, reacting 16-dehydropregnenolone acetate oxime with p-toluenesulfonamide chloride, benzenesulfonyl chloride, triethylamine or N,N-dimethyl-pyridine (DMAP) in a dichloromethane solution, concentrating the solvent after reaction, adding methanol and a sodium hydroxide solution for refluxing hydrolysis, cooling and regulating the pH value to 7-8, adding activated carbon, then refluxing for 30-60 minutes, filtering, concentrating, crystallizing, and centrifuging to obtain the product. The synthesis method provided by the invention is simple to operate, and has characteristics of mild reaction conditions, high yield, low environmental pollution and the like.
Owner:HUNAN KEREY BIOTECH
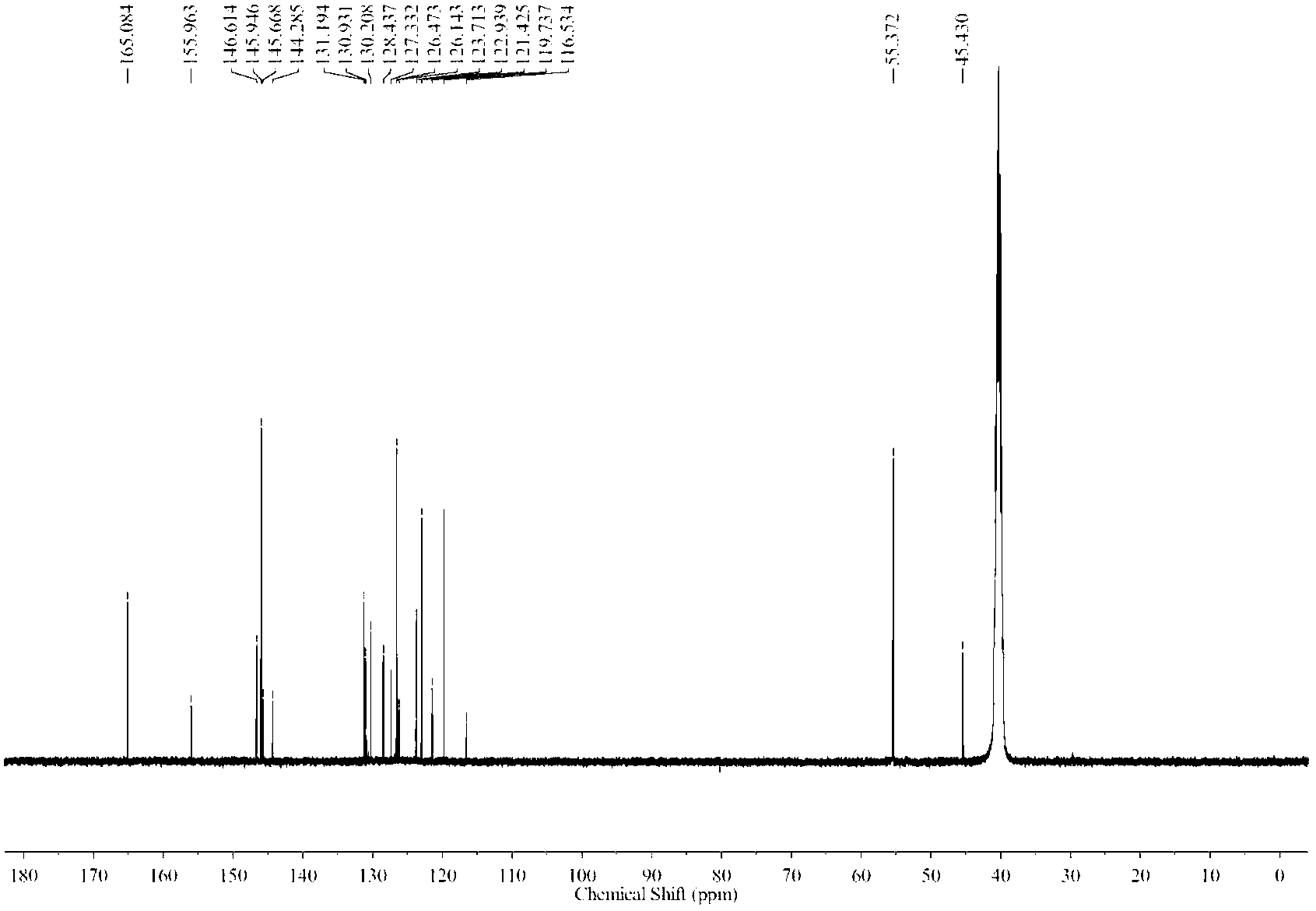
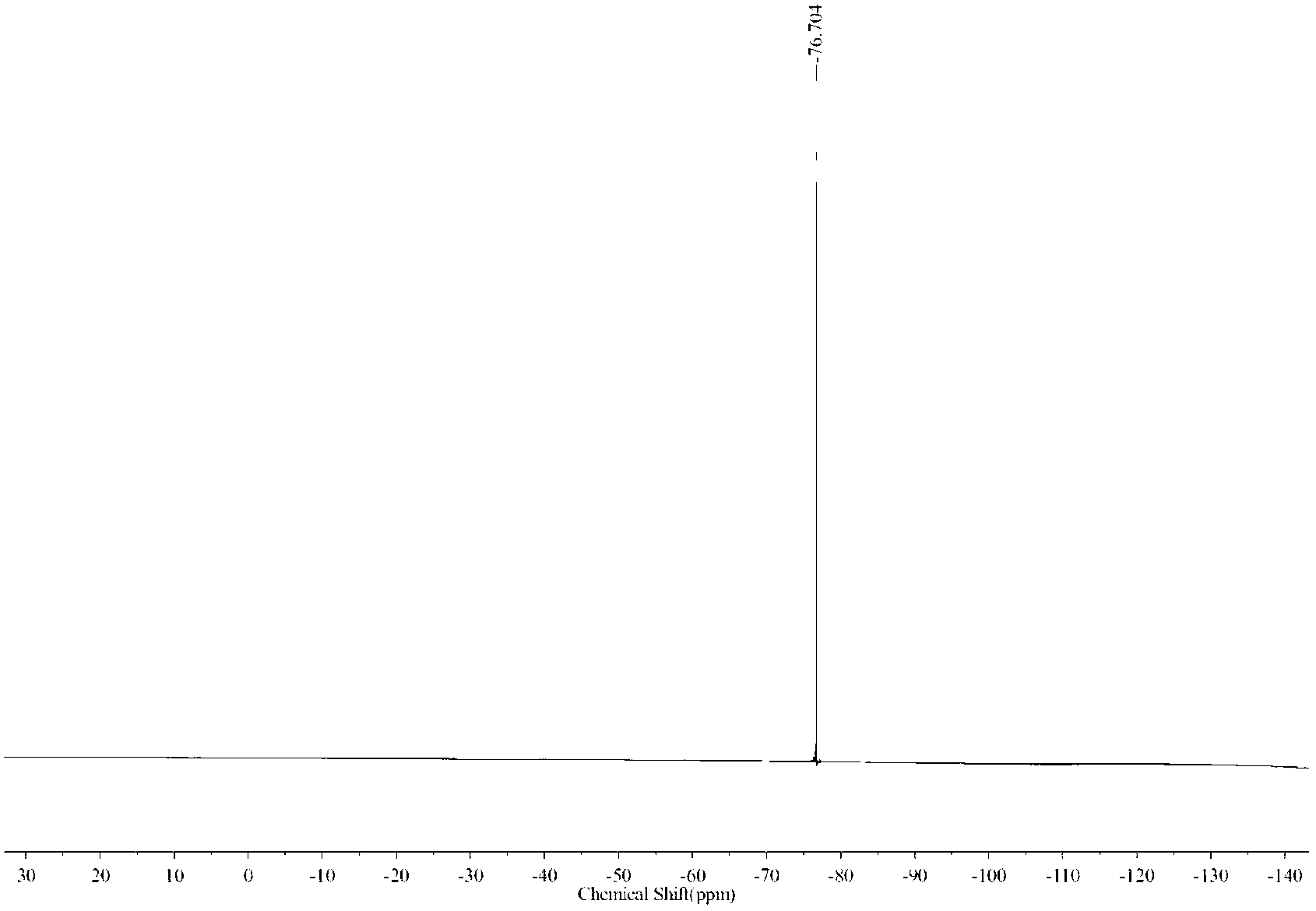
Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide
The invention provides a preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C]pyrimidine-2-)benzsulfamide, comprising the following steps: using a compound represented by formula (IV) as an initial raw material to synthesize a compound represented by formula (III); synthesizing the compound represented by formula (II); finally synthesizing a target compound represented by formula (I). In the invention, two phenolic alcohols are jointed to form ether, thereby preventing from using costly non-marketization 2,2-difluorobromoethane for introducing difluoroethoxy as a reagent; in the process of synthesizing target product, besides 3,5-dimethyl pyridine and dimethyl sulfoxide, a catalytic amount of 18-crown-6 crown ether is used to improve the reaction timeand reaction yield. The invention simplifies the reaction conditions, optimizes the synthesis process, enhances the reaction yield, and reduces production cost, thereby greatly improving the synthesis effect. The reaction formula is represented as follows.
Owner:孙智华 +2
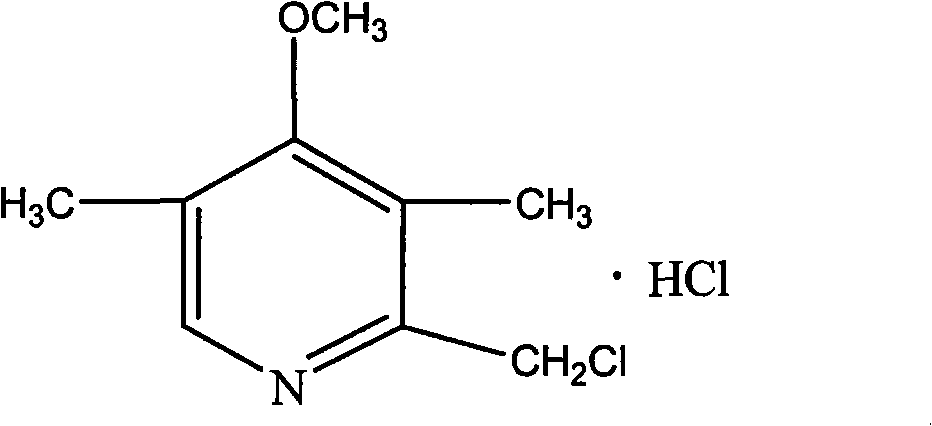
Purifying method of 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride
The invention relates to a purifying method of 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride, and the method comprises the following steps: adopting 3,5-dimethylpyridine as raw material to prepare 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride through the steps of oxidation, nitration, methoxylation, methylation, oxidation and chloro substitution or adopts 2,3,5-trimethylpyridine as raw material to prepare 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride through the steps of oxidation, nitration, methoxylation, acetylation, hydrolyzation and chloro substitution, removing the solvent of the obtained 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride reaction solution though the chlorination reaction, washing with organic solvent, filtrating and drying to obtain solid 2-chloromethyl-4-methoxyl-3,5-dimethylpyridine chloride. The purity of the product obtained by the method of the invention can reach above 99%, the operation is simple, and the method reducesthe cost of the prior art and is beneficial for industrial production.
Owner:JIANGSU ZHONGBANG PHARMA
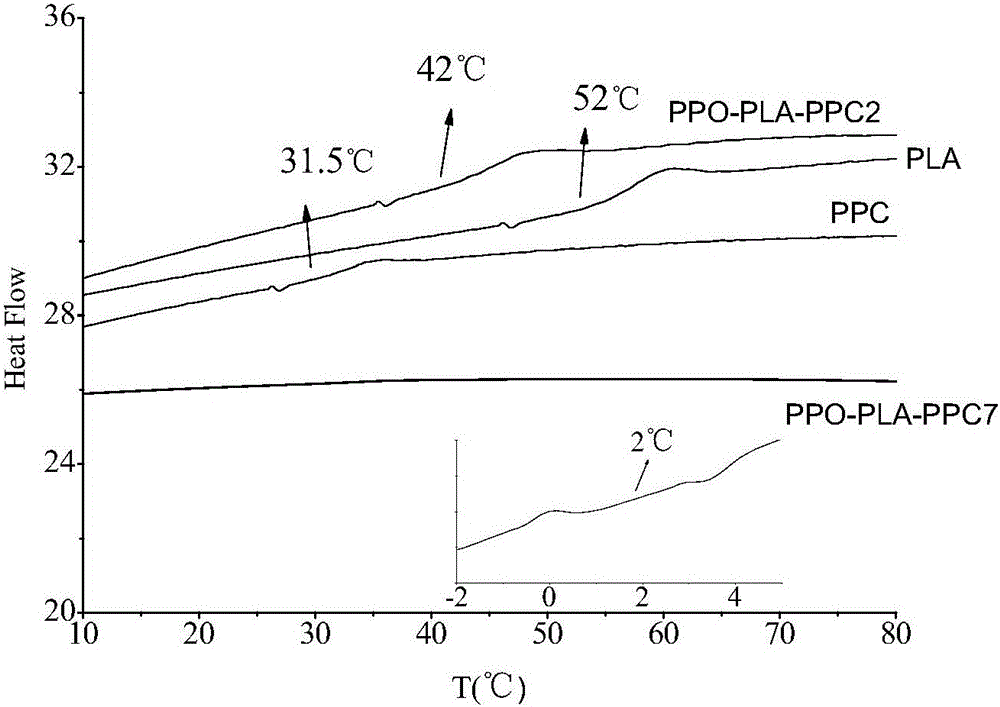
Composite catalyst for preparing polyether-polylactide-aliphatic polycarbonate ternary block copolymer and application of composite catalyst
The invention relates to a composite catalyst for preparing a polyether-polylactide-aliphatic polycarbonate ternary block copolymer and an application of the composite catalyst. The composite catalyst comprises a metal Salen catalyst and a cocatalyst in a molar ratio of 1: (0.5-2), wherein the cocatalyst is bis-(triphenylphosphine)iminium chloride, 4-dimethylaminopyridine or 2,6-lutidine. The composite catalyst has the following beneficial effects: 1, The catalysis effect of the composite catalyst is good, the catalysis efficiency is high, the obtained ternary copolymer has larger number-average molecular weight and narrow molecular weight distribution; 2, composition of a polyether segment, a polylactide segment and a PPC (poly(propylene carbonate)) segment in the copolymer can be effectively controlled through regulation of step reaction time and monomer ratio, the glass transition temperature of the copolymer is 2-42 DEG C, the elongation at break is 55%-331%, and the tensile strength is 6-39 MPa.
Owner:WUHAN UNIV OF TECH
Preparing process of Ru(II) polypyridine complex
InactiveCN1986555AGroup 8/9/10/18 element organic compoundsMicrobiological testing/measurementSodium tetrafluoroboratePhenanthroline
The preparation process of Ru(II)-polypyridine complex includes the following steps: preparing 1,10-phenanthroline-5,6-diquinone, pyridine [3, 2-a:2', 3'-c] phenazine as ligand I, and 7,8-dimethyl pyridine [3, 2-a: 2', 3'-c] phenazine as ligand II, Ru(bipy)2Cl2.2H2O (bipy=2, 2'-dipyridyl) as precursor I and Ru(phen)2Cl2.2H2O as precursor II; adding certain amount of Ru(bipy)2Cl2.2H2O / Ru(phen)2Cl2.2H2O, ligand I / ligand II, and mixture liquid of methanol and water, heating reflux for certain time, heating to concentrate, adding water and boiling, freezing, adding sodium tetrafluoborate, filtering and separating out precipitate, re-crystallization in alcohol, and stoving under infrared lamp to obtain the Ru(II)-polypyridine complex. The Ru(II)-polypyridine complex is used as nucleic acid recognizing probe for the analysis and detection of nucleic acid, and has high stability, high sensitivity, high selectivity and other advantages.
Owner:WUHAN UNIV
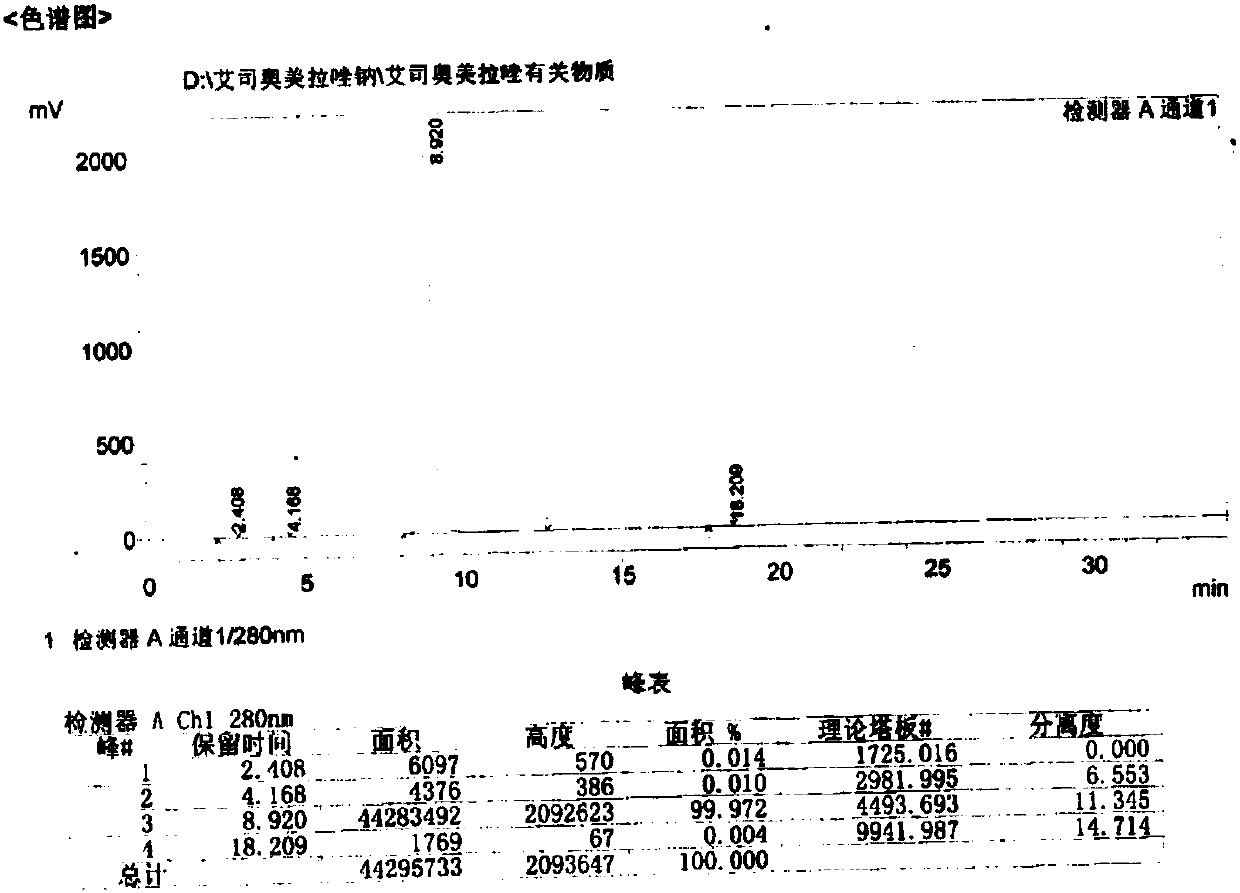
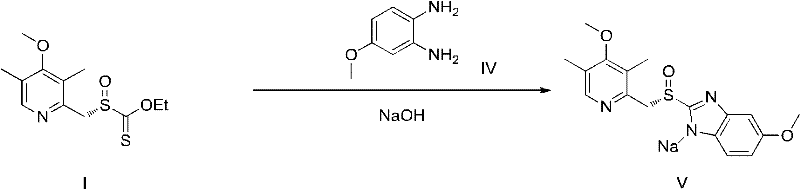
Omeprazole sodium and preparation method
ActiveCN103204841APrevent reflux combined with aspirationPrevent rebleedingOrganic chemistryOmeprazole Sodium4-methoxypyridine
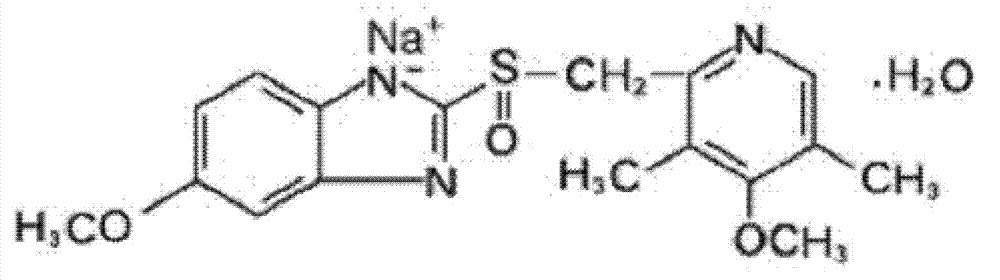
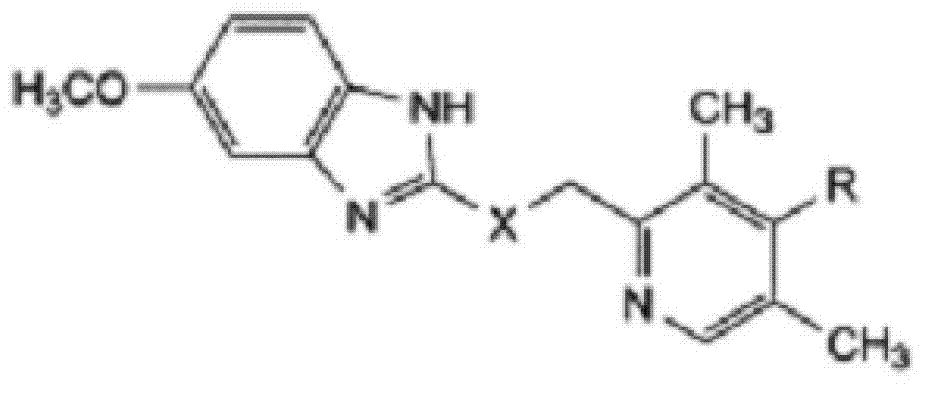
The invention relates to omeprazole sodium and a preparation method, and in particular relates to a method for preparing omeprazole or a salt thereof. The method comprises the following steps of: (1) reacting 2-chlorinated methyl-3,5-dimethyl-4-methoxypyridine or a salt thereof and 2-mercapto-5-methoxy-1H-benzimidazole to generate 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridine-2-yl)methylmercapto]-1H-benzimidazole; and (2) oxidizing 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridine-2-yl)methylmercapto]-1H-benzimidazole to generate omeprazole. The invention further relates to omeprazole prepared by the invention or a sodium salt thereof. A product prepared by the method has high purity.
Owner:CHENGDU TIANTAISHAN PHARMA
Fluorescence chemical sensor and preparation method and application thereof
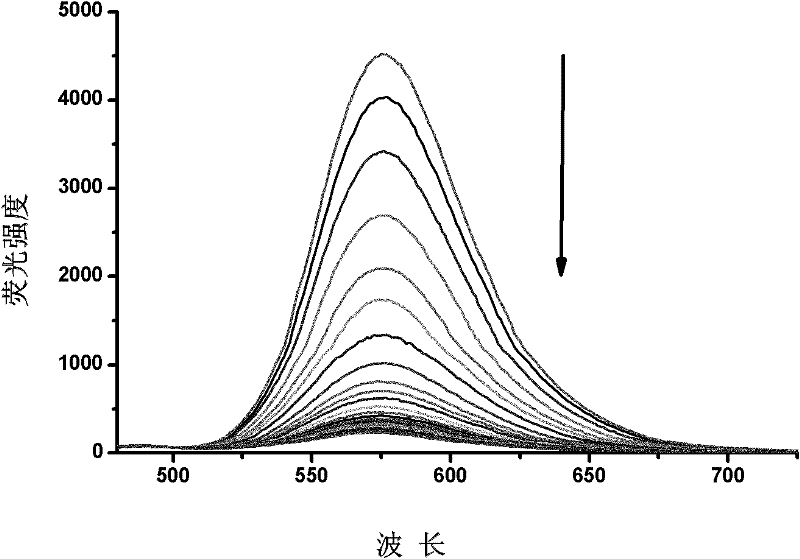
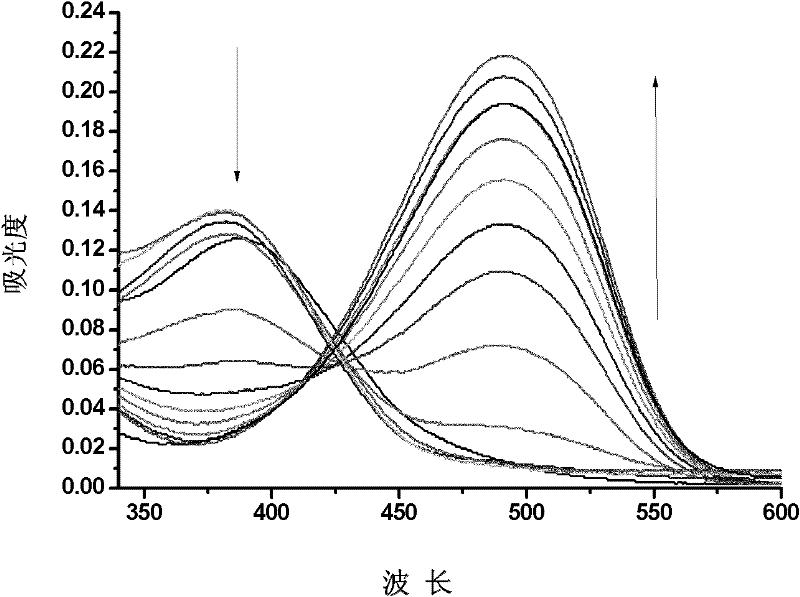
InactiveCN102190670AGood water solubilityObvious color changeChemiluminescene/bioluminescenceCopper organic compoundsFluorescenceBenzaldehyde
The invention provides a fluorescence chemical sensor and a preparation method and application thereof. The structural formula of the fluorescence chemical sensor is shown as the formula (I), wherein R1 refers to sulfur or oxygen; and R2 refers to sulphonate, iodate, fluorphosphate or fluoborate. The preparation method for the fluorescence chemical sensor comprises the following steps of: (1) performing oxidation reaction on 4'-(N,N-dimethyl pyridyl amido)benzene under the action of an oxidant to obtain 4'-(N,N-dimethyl pyridyl amido)benzaldehyde; (2) reacting the 4'-(N,N-dimethyl pyridyl amido)benzaldehyde with a compound in a formula (III) to obtain a compound in a formula (II); and (3) reacting the compound in the formula (II) with copper ion-containing inorganic salt to obtain the fluorescence chemical sensor, wherein in the formulas (II) and (III), R1 refers to sulfur or oxygen; and R2 refers to sulphonate, iodate, fluorphosphate or fluoborate. The fluorescence chemical sensor can specifically, efficiently, timely and easily detect cyanide ions in aqueous solution.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI +1
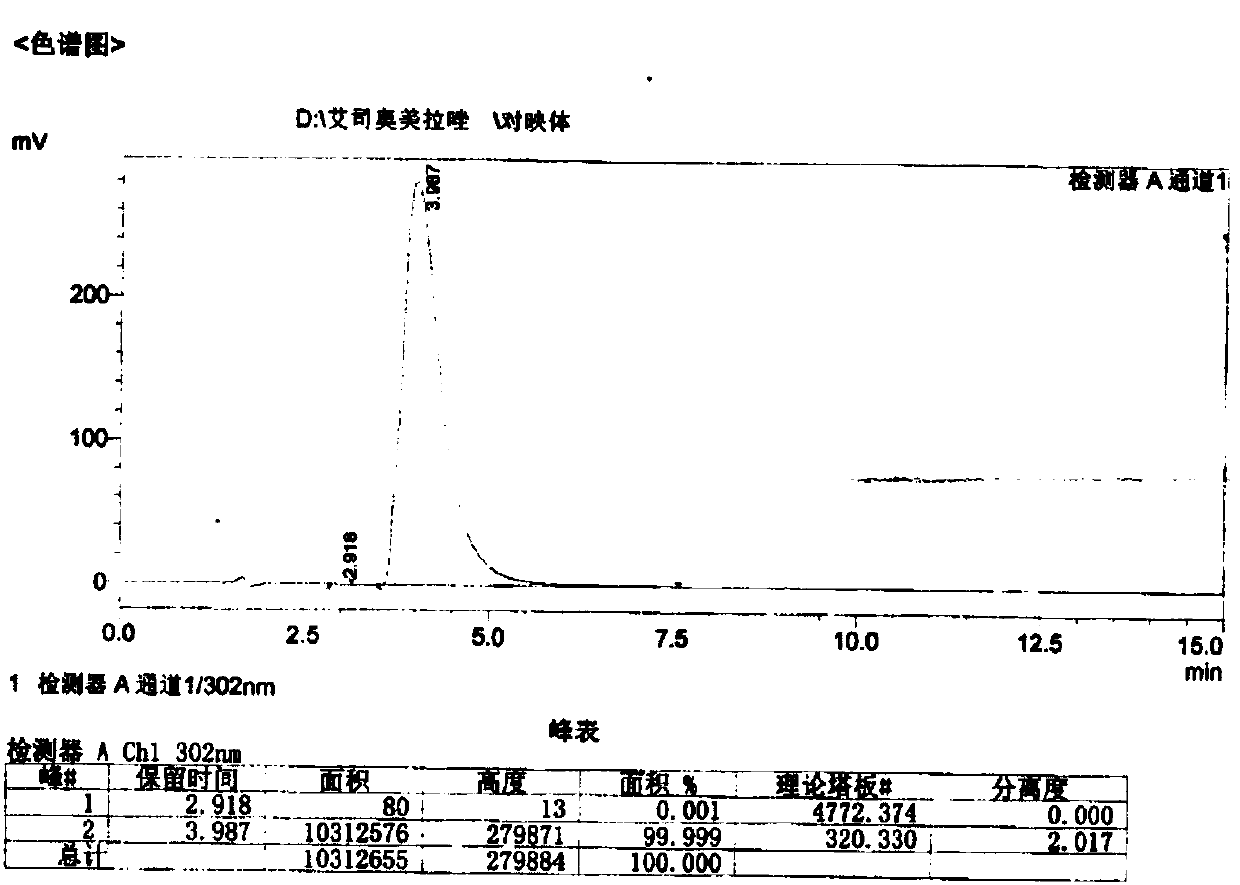
Preparation method of esomeprazole and preparation method of esomeprazole salt
The invention adopts 2-chloromethyl-4-nitryl-3, 5-dimethyl pyridine hydrochloride and 5-methoxyl-2-mercapto benzimidazole as starting materials to prepare esomeprazole salt by condensation, asymmetric oxidation and methoxidation. A preparation method has the advantages that the repeatability is good, the operation is simple, and the industrial production is easy; and the preparation conditions are moderate, the generation of impurities such as nitric oxide and sulphone is reduced in the preparation process, and the yield and the purity of the esomeprazole salt are increased.
Owner:SHANDONG UNIV +1
Functional isotactic polypropylene and preparation method thereof
InactiveCN103483482AImprove copolymerization performanceImprove tolerancePolymer scienceRegioselectivity
The invention provides functional isotactic polypropylene and a preparation method thereof. The functional isotactic polypropylene is prepared through polymerizing polymerization monomers including propylene and terminal-halogenated 1-olefin under the action of a catalyst and a catalyst promoter, wherein the catalyst is a dimethyl pyridine amine-hafnium complex, and the catalyst promoter is triphenylmethyl tetrakis-(pentafluorophenyl)-borate. The catalytic systems of the catalyst and the catalyst promoter have high three-dimensional regioselectivity for the polymerization monomers and have excellent copolymerization capacity for long-chain halogenated 1-olefin, so that the prepared functional isotactic polypropylene has relatively high regularity and high isotacticity ([mmmm]) of over 99%.
Owner:应忠
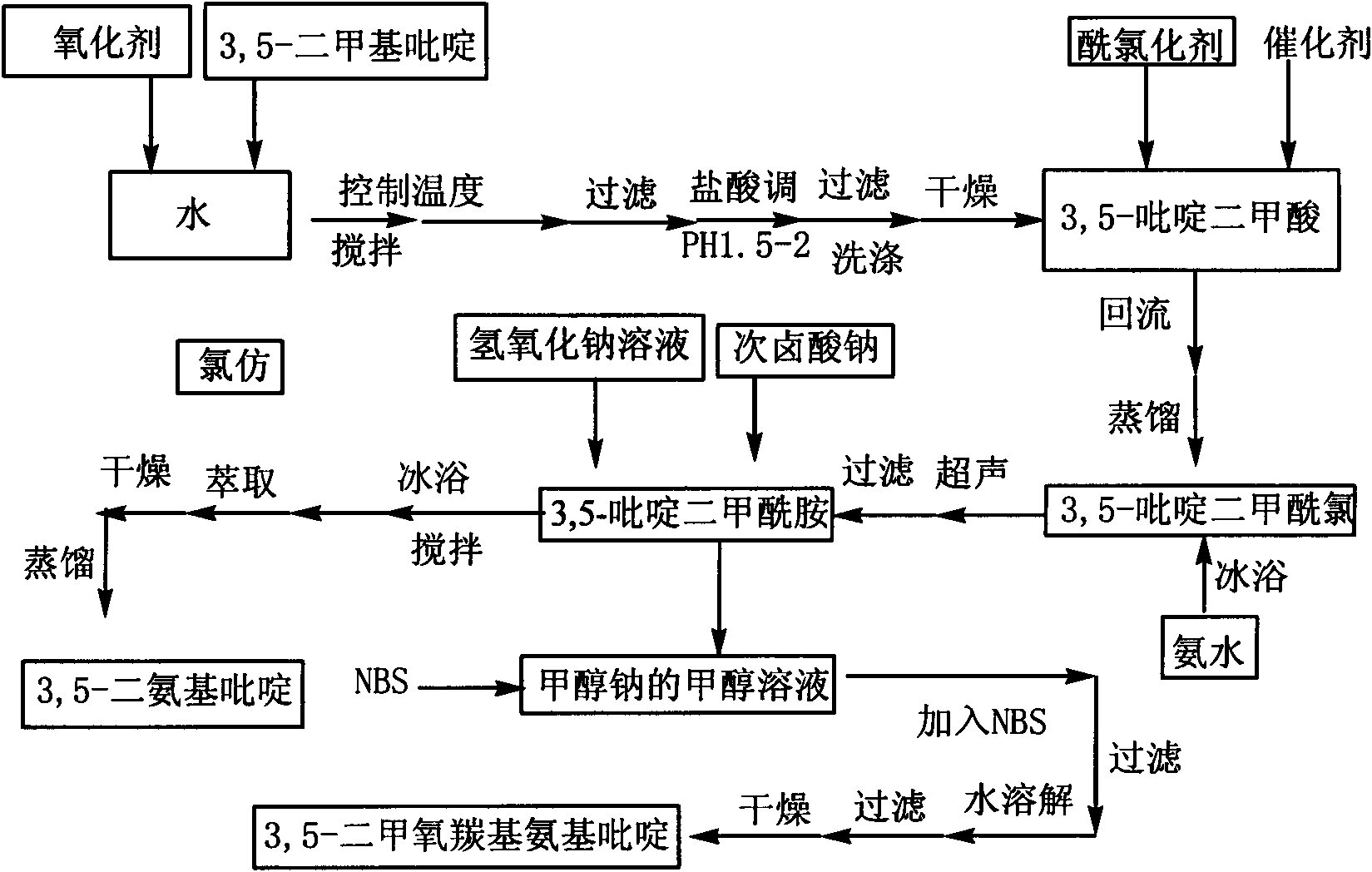
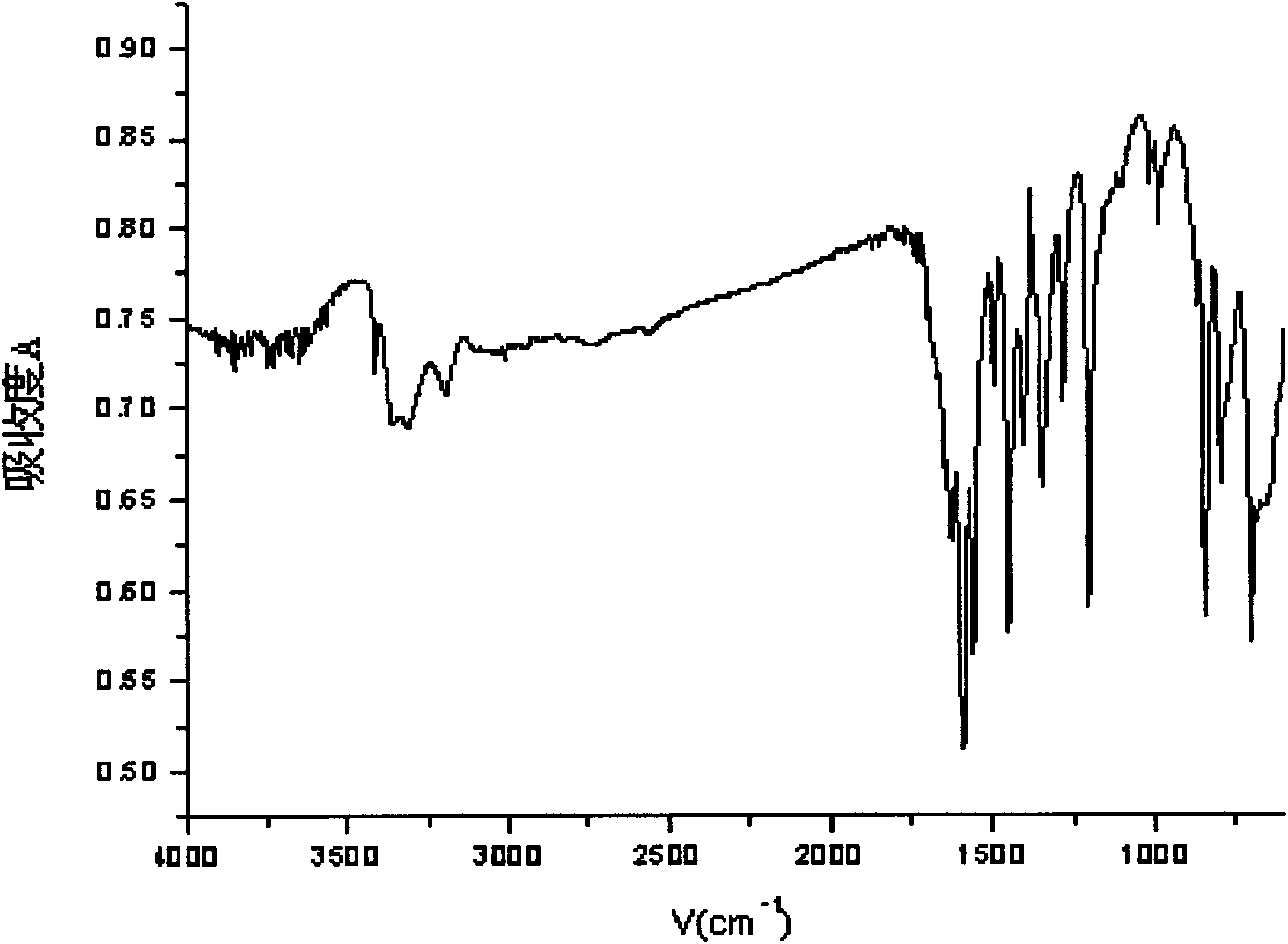
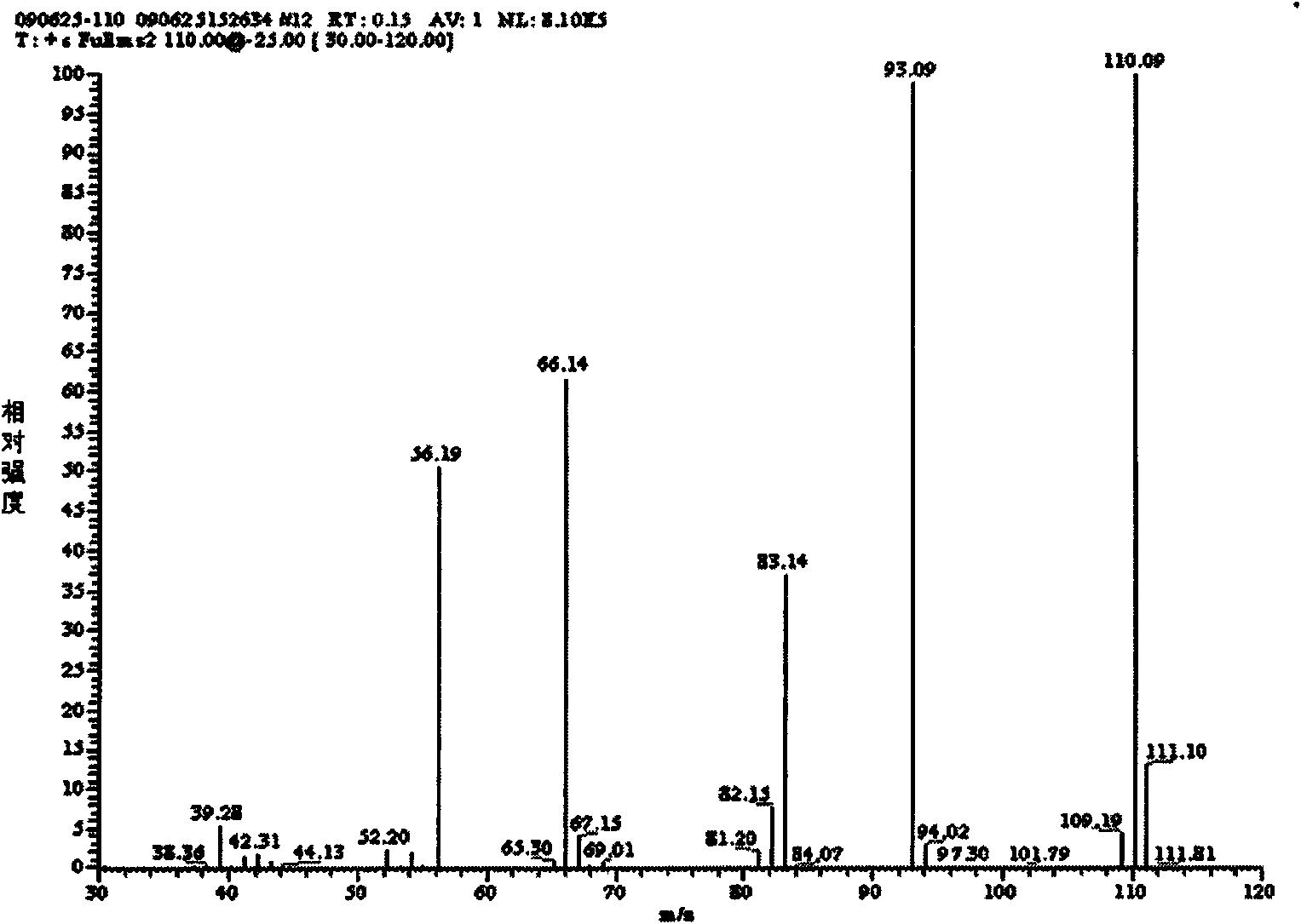
3,5-diaminopyridine and preparation method of 3,5-dimethoxycarboxylaminopyridine
The invention discloses 3,5-diaminopyridine and a preparation method of 3,5-dimethoxycarboxylaminopyridine. The preparation method comprises the following steps: using 3,5-dimethylpyridine to perform oxidation reaction so as to obtain 3,5-pyridinedicarboxylic acid; using 3,5-pyridinedicarboxylic acid to perform acyl chlorination and obtain 3,5-pyridinedicarbonyl chloride; using 3,5-pyridinedicarbonyl chloride to perform ammoniation reaction to obtain 3,5-pyridinedimethylformamide; and performing Hofmann degradation on the 3,5-pyridinedimethylformamide to prepare 3,5-diaminopyridine or 3,5-dimethoxycarboxylaminopyridine. In the invention, 3,5-dimethylpyridine is used as raw material to design the synthetic route of the target compound, the reaction conditions of each step are mild, the product purity and yield are obviously increased, the yield is more than 70%; and the aftertreatment of each step of the provided synthetic route is simple, the method is green and environmentally friendly, the oxidation product is solid manganese dioxide, the oxidation product can be recycled through filtering, and the compound 2 can be used to perform the next step without being separated.
Owner:NANJING UNIV OF SCI & TECH
Preparation method of biapenem aseptic powder
The invention relates to a preparation method of biapenem aseptic powder. Specifically, in a preparation process of a biapenem crude product, an alkaline substance of 2,6-lutidine is used so that post-reaction treatment is simple and a yield is high. In a preparation process of biapenem aseptic powder, acetic acid is utilized for adjustment of a pH value of water so that dissolvability and stability of biapenem in water are improved. Biapenem aseptic powder prepared through the preparation method has a high yield and controllable quality.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Phosphoramidite monophosphine ligand and preparation method and application thereof
ActiveCN101445517AEasy to synthesizeOrganic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsAlkaneCycloaddition
A phosphoramidite monophosphine ligand has the structural formula thereof shown above, wherein R<1> or R<2> is selected from H, methyl or ethyl, and R<3> is selected from aryl or C1-C6 alkyl. The preparation method of the phosphoramidite monophosphine ligand comprises the following steps: adding dialkylphosphine chloride or diarylphosphine chloride of dichloromethane, triethylamine and C1-C6 alkane to a container containing enantiomer pure 4,4'6,6'-tetrakis-trifluoromethyl-biphenyl-2,2'-diamine (TF-BIPHAM) or enantiomer pure 3,3'-dibromo-4,4'6,6'-tetrakis-trifluoromethyl-biphenyl-2,2'-diamine in the presence of N2, stirring at room temperature for 12 to 72 h, removing solvent under reduced pressure, and purifying with column chromatography. The phosphoramidite monophosphine ligand is used in asymmetric [3+2] 1,3-dipolar cycloaddition of azomethine ylide, resulting in good effect and high enantiomeric selectivity of product (up to 99%).
Owner:常熟紫金知识产权服务有限公司
Process of separating methyl pyridine mixture
The present invention relates to process of separating mixture of methyl pyridine isomer and / or homologous compound. The separating process includes the main steps of: normal pressure rectification of the methyl pyridine mixture in the presence of C1-C6 monobasic alcohol water solution as the first azeotropic agent and collecting the fraction comprising the required component and the first azeotropic agent; and the subsequent normal pressure azeotropic rectification of the collected fraction with benzene as the second azeotropic agent to obtain the target product. The present invention can obtain components with purity not lower than 99 wt%, and has cheap material, easy control and low cost.
Owner:EAST CHINA UNIV OF SCI & TECH +1
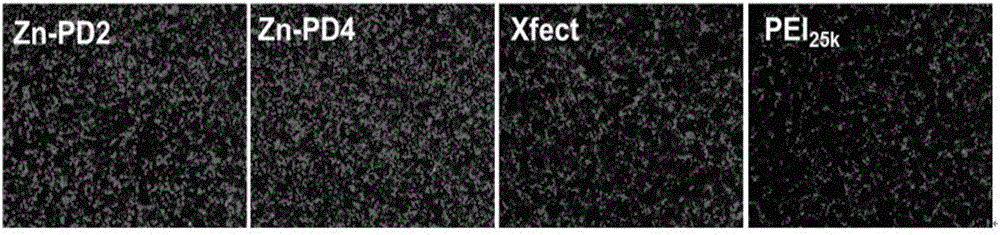
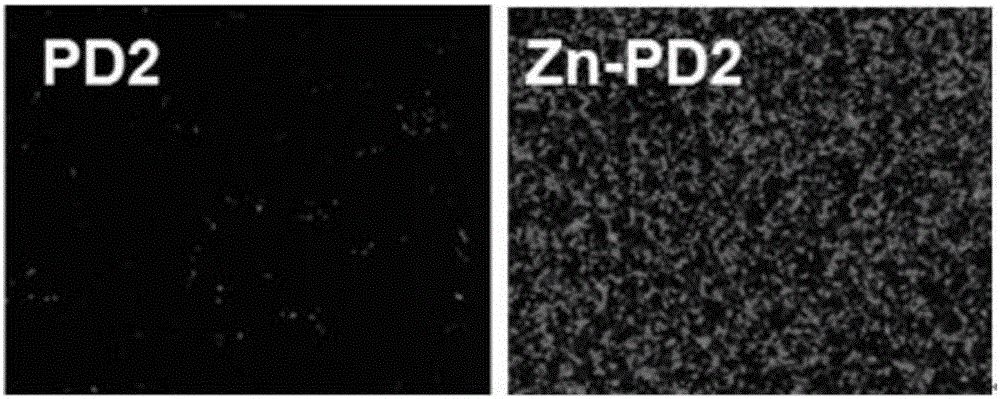
Metal coordination polycation gene vector and preparation method and application thereof
ActiveCN106496555AImprove bindingIncrease forceOther foreign material introduction processesStimuli responsiveCell membrane
The invention discloses a metal coordination polycation gene vector. The metal coordination polycation gene vector consists of a coordinated metal, a coordinated unit, a stimuli-responsive unit and polycations, and the structural general formula of the metal coordination polycation gene vector is as shown in the specification, wherein M represents the coordinated metal; a dimethyl pyridylamine component represents the coordinated unit; a disulfide bond component represents the stimuli-responsive unit; a ball represents the polycations; n represents the quantity of metal ion ligands grafted by one polycation molecule, n is 1 to 200; and the metal coordination polycation gene vector is used for non-virus gene vectors. The metal coordination polycation gene vector has the advantage that the metal ion ligands containing stimuli-responsive radicals are modified on the polycations, so that the DNA binding capacity of the polycations and the acting force with cytomembranes are greatly improved; after the compound enters cells, the metal ion ligands and the polycations are disconnected, DNAs are released and expressed so as to show extremely high transfection efficiency, so that the application of the polycations in the aspect of the non-virus gene vectors is greatly expanded due to metal coordination monomers in the invention.
Owner:NANKAI UNIV
Preparation method for esomeprazole
InactiveCN107892683AHigh yield and purityReduce generationOrganic chemistry methodsNitrogen oxideSulfone
The invention discloses a method for preparing esomeprazole, belonging to the technical field of medicine. According to the invention, 2-chloromethyl-4-methoxy-3,5-dimethylpyridine hydrochloride and 2-mercapto-5-methoxybenzimidazole are used as starting materials and are subjected to condensation and oxidation so as to prepare an esomeprazole salt. The method has good reproducibility, and is simple to operate, easy for realizing industrial production and mild in preparation conditions; and the production of impurity nitrogen oxide and sulfone (peroxide) during the preparation is reduced, and the yield and purity of the esomeprazole salt are improved.
Owner:JIANGSU ZHONGBANG PHARMA
A synthetic method of amikacin
InactiveCN105440090AReduce manufacturing costEasy to operateSugar derivativesSugar derivatives preparationAmikacinSilylene
A synthetic method of amikacin is disclosed. Gamma-4-phthalimido-2-hydroxy butyric acid is adopted as a raw material for direct acylation. 4-dimethylaminopyridine (DMAP) or 1-hydroxybenzotriazole (HOBT) is adopted as a catalyst. N,N'-dicyclohexylcarbodiimide is adopted as a condensing agent. Silyl kanamycin A is directly acylated to obtain an acylation product and the acylation product is subjected to acidolysis and hydrazinolysis to obtain the amikacin. A production operation for specially preparing active ester for acylation is not needed in the method, thus simplifying operation steps and production equipment. N-hydroxyphthalimide for preparing the active ester is not used in the direct acylation, thus reducing the production cost. By optimization of acylation conditions, selectivity of the acylation is improved, the synthesis yield is ensured, contents of impurities is lowered, and beneficial conditions for subsequent purification of the amikacin are provided.
Owner:CHONGQING DAXIN PHARMA +2
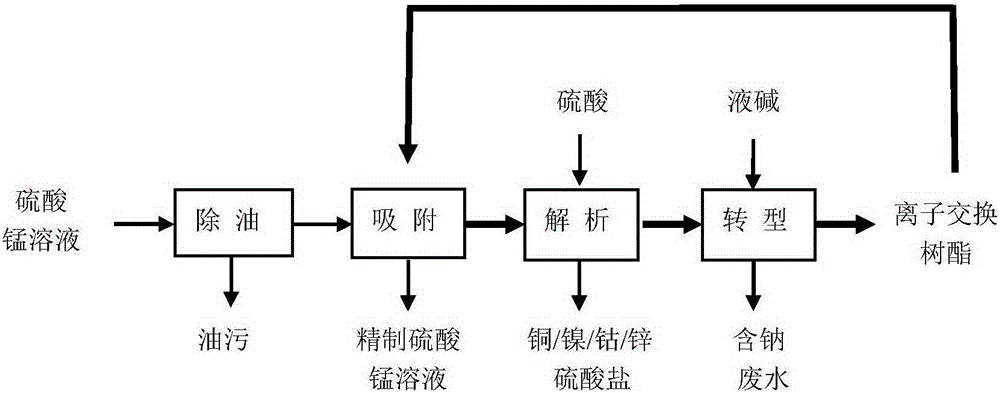
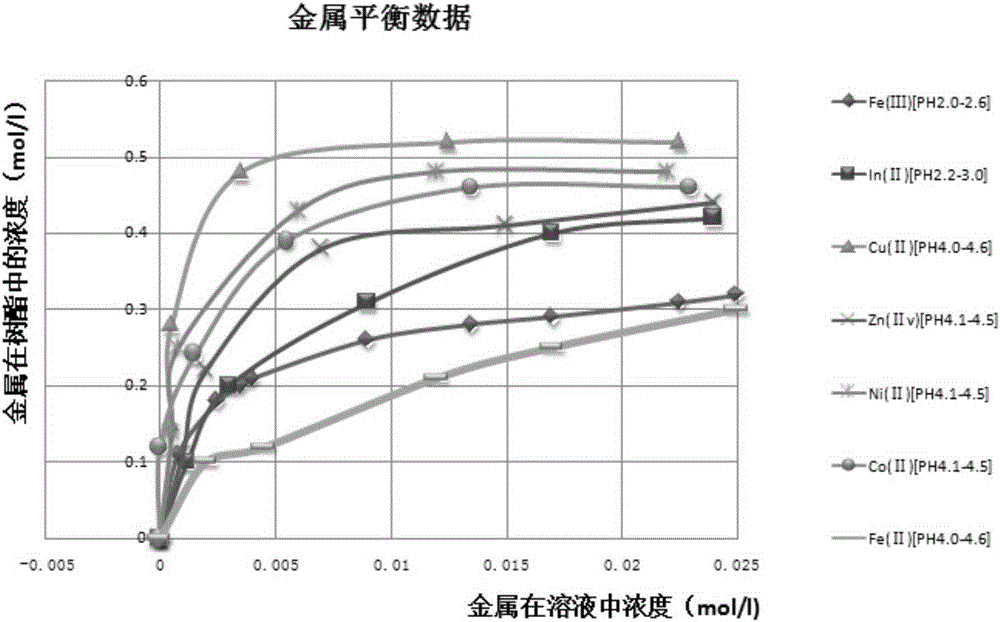
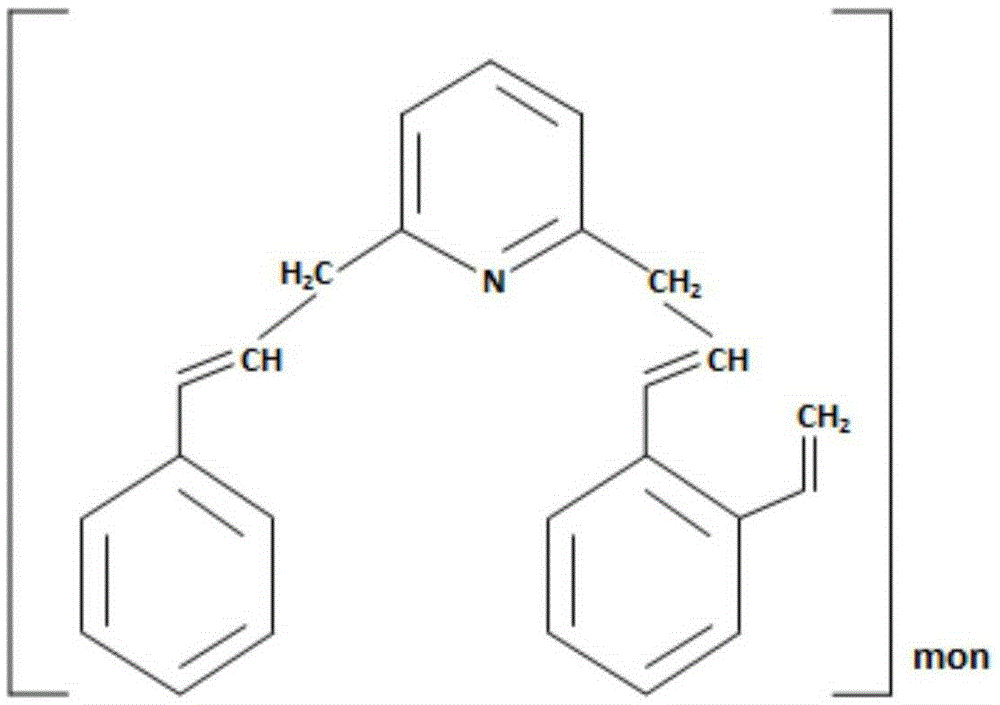
Method for purifying manganese sulfate solution
The invention discloses a method for purifying a manganese sulfate solution. The method is characterized by comprising the steps that ion exchange resin is used for treating the manganese sulfate solution, and is chelate resin with a lutidine group. The manganese sulfate solution is output through a Ni-Co wet method metallurgical extraction procedure, and due to the fact that copper, zinc, nickel, cobalt and other impurities are included in the manganese sulfate solution, the manganese sulfate solution is treated as waste liquid, resources are wasted, and environment-friendly treatment cost is increased. According to the method, the ion exchange process is used for adsorbing and removing impurity metal elements, an ion exchange column is filled with chelate resin with a Purolite<>S960 group, and according to the selective sequence under the same condition, Cu>Ni>Co>Pb>Zn>Cd>Fe<+3>>Mn>Mg>Ca>Na. Certain conditions are controlled, copper, nickel, cobalt, zinc and other metal ions can be selectively adsorbed and removed, and the pure manganese sulfate solution is obtained.
Owner:广西银亿再生资源有限公司 +1
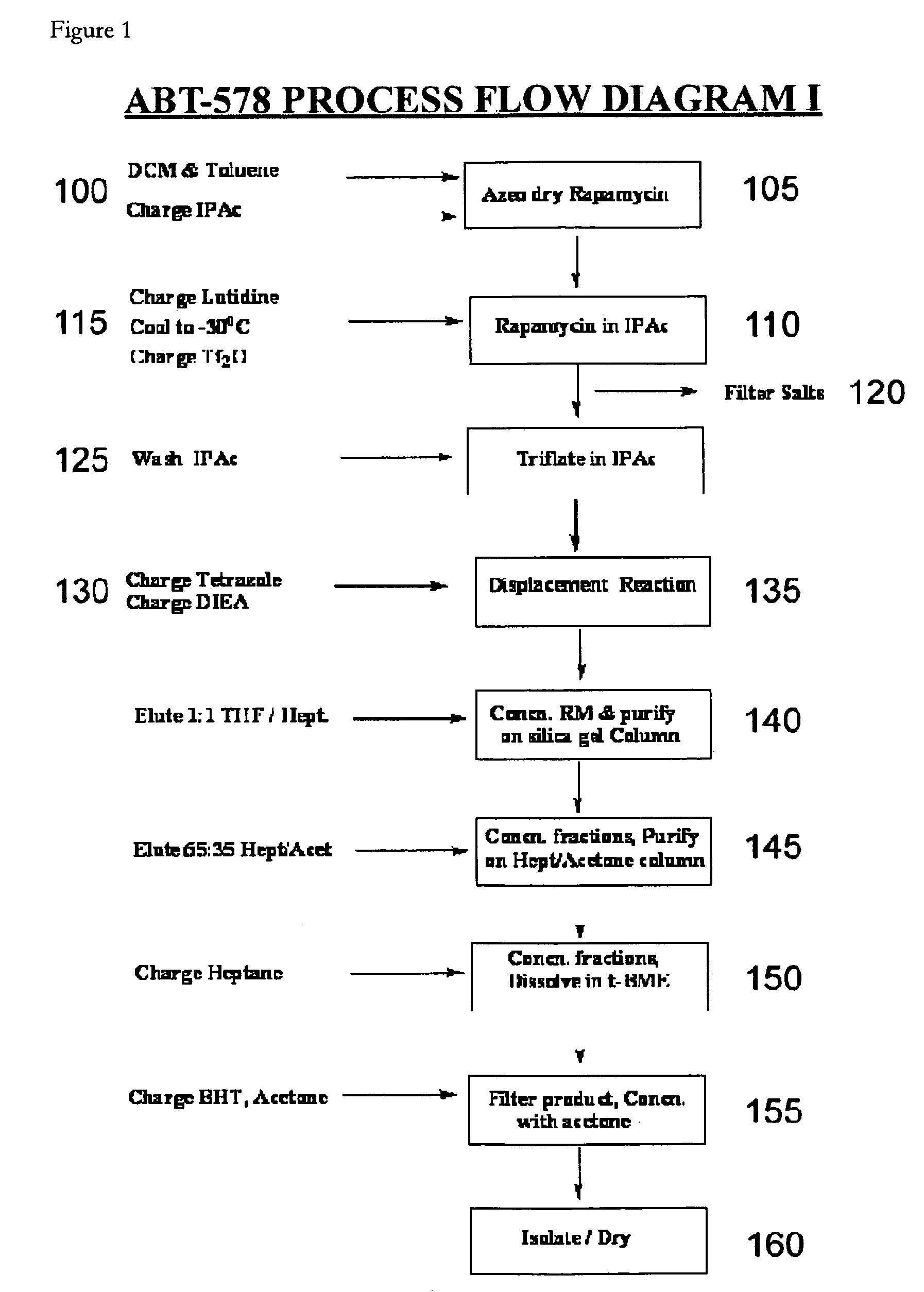
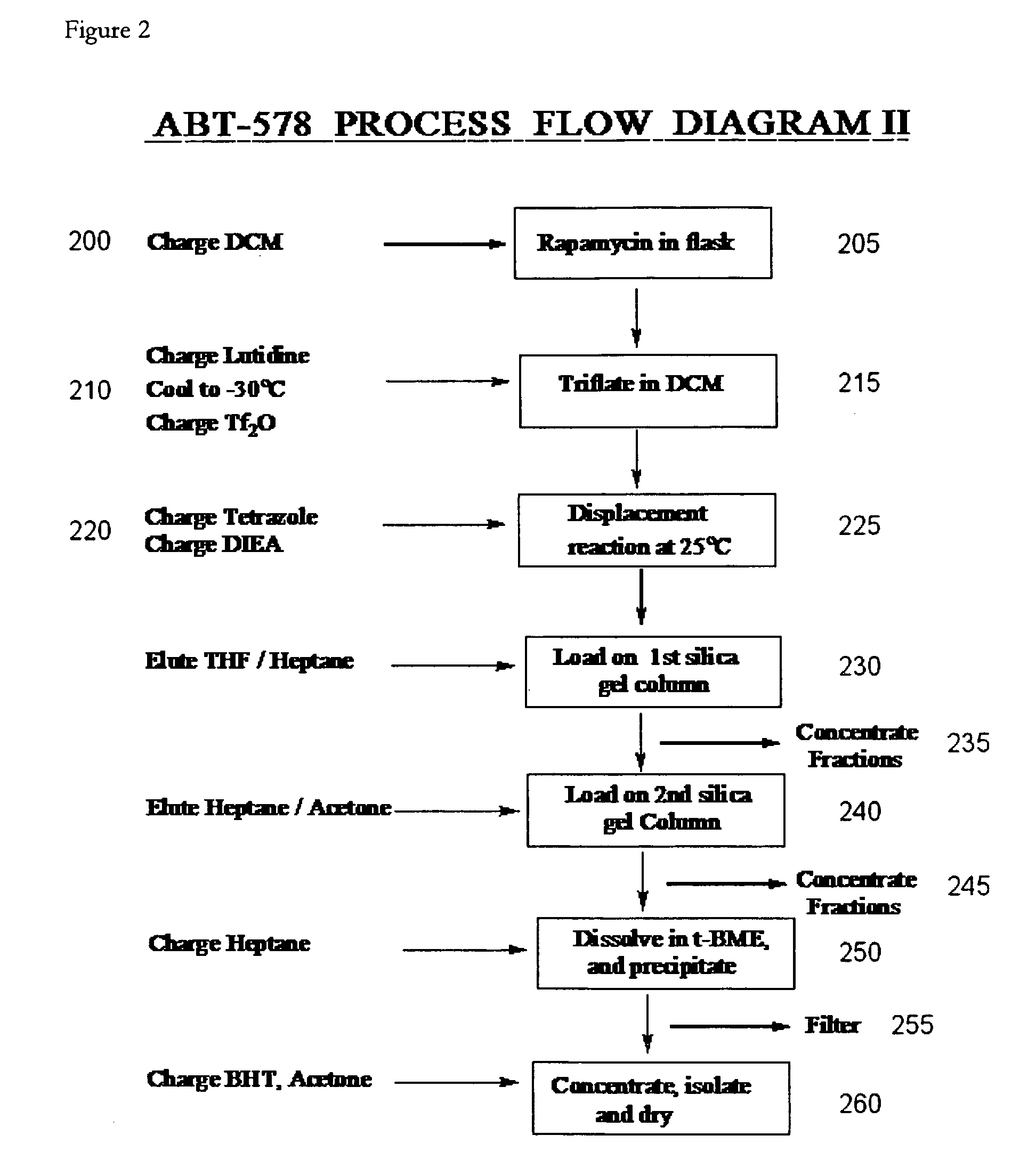
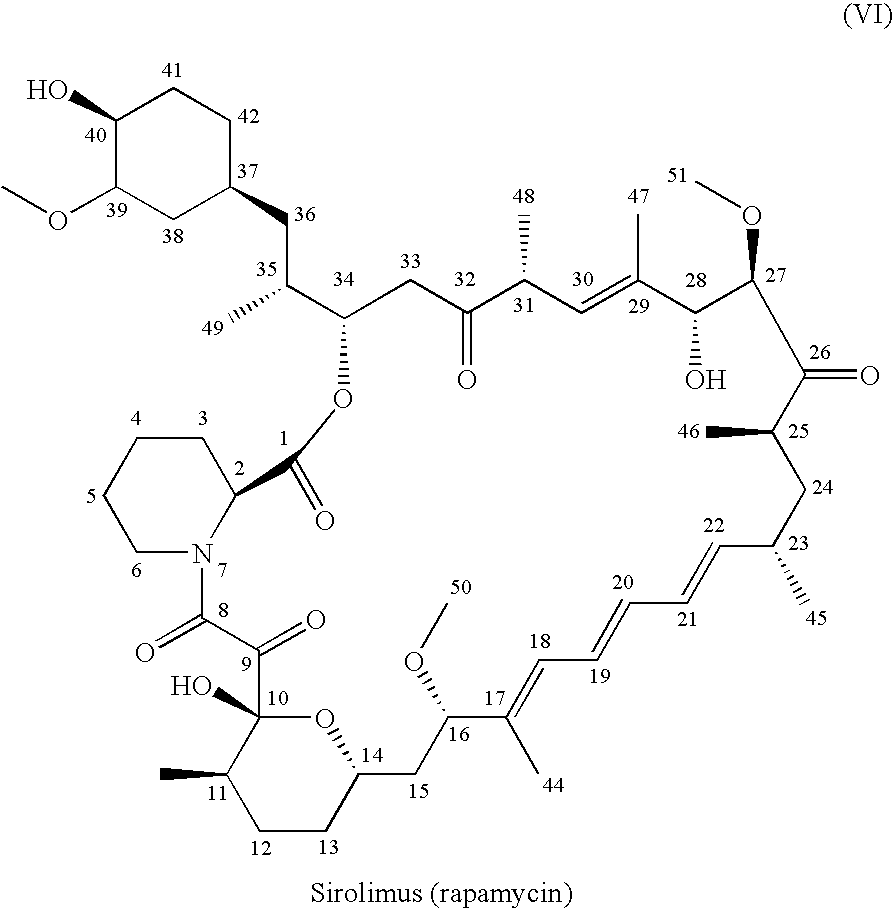
One pot synthesis of tetrazole derivatives of rapamycin
A single-step, one-pot process to obtain zotarolimus and other rapamycin derivatives on large scale is presented, which improves currently available synthesis schemes. In one embodiment, dried rapamycin is dissolved in isopropylacetate (IPAc). The solution is cooled, and 2,6-Lutidine is added, followed slowly adding triflic anhydride at −30° C. Salts are then removed by filtration. Tetrazole, followed by a tert-base diisopropylethylamine (DIEA) is added to the triflate solution. After incubation at room temperature, the product is concentrated and purified by a silica gel column using THF / heptane as eluant. The fractions containing the product are collected, concentrated, and purified again using an acetone / heptane column. The product containing fractions are concentrated. The product is dissolved in t-BME and precipitated with heptane. The solids are dissolved in acetone, treated with butylated-hydroxy toluene (BHT), and the solution concentrated. The process is repeated twice with acetone to remove solvents. At least one stabilizing agent is added, such as BHT at 0.5% before drying.
Owner:ABBOTT LAB INC
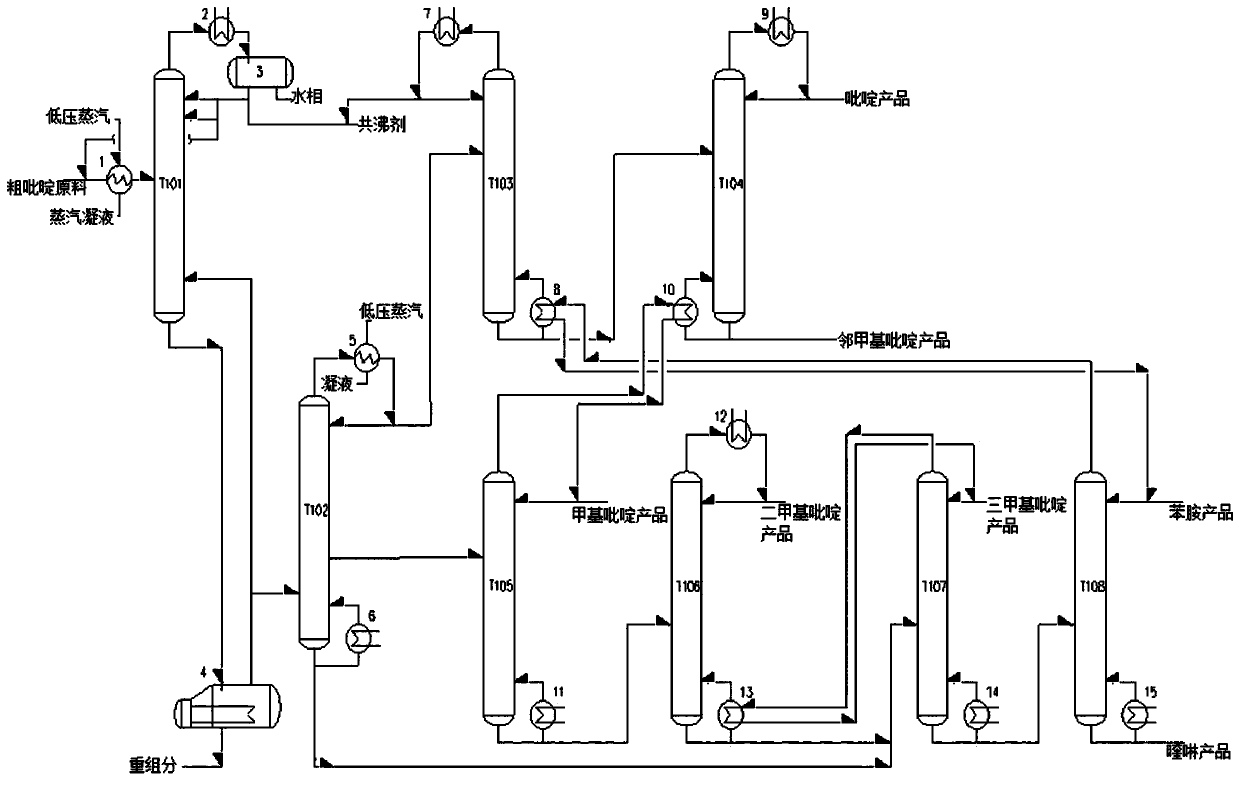
Method and device for preparing high-purity pyridine series products from crude pyridine by refining
ActiveCN109912500ASimple processSimplify equipmentOrganic chemistryChemical industryGas phaseEvaporation
The invention discloses a method for preparing high-purity pyridine series products from crude pyridine by refining. The method comprises following steps: (1), drying: a crude pyridine raw material and an entrainer are mixed and preheated to enter a drying tower for azeotropic distillation, water phase and the entrainer are obtained at the top of the drying tower, the entrainer is recycled or extracted for standby application, and a crude pyridine material is obtained at the bottom of the drying tower; (2), heavy component removal: the crude pyridine material obtained in step (1) enters an evaporation kettle and is subjected to the heavy component removal treatment, part of gas phase at the top of the evaporation kettle returns to the drying tower, and the other part enters a pre-fractionation tower and is subjected to light and heavy material fractionation; (3), distillation of the pyridine series products. The pyridine series products with high added value are separated out with adoption of azeotropic distillation, atmospheric and vacuum distillation and heat integration technologies, purity of pyridine, o-methylpyridine, methylpyridine, dimethyl pyridine and trimethyl pyridine reaches 96wt% or higher, and one energy-saving and high-efficiency technical method for preparing the high-purity pyridine series products from crude pyridine by refining is obtained.
Owner:CHINA CONSTR IND & ENERGY ENG GRP CO LTD
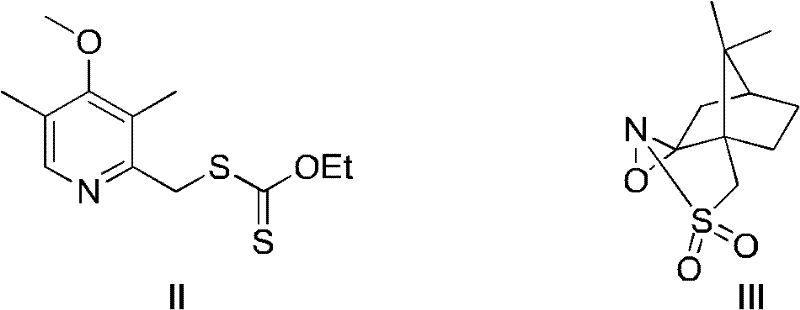
Novel chiral sulfoxide compound and method for preparing esomeprazole by using novel chiral sulfoxide compound
The invention discloses a (S)-(((4-methoxy-3,5-dimethyl pyridine-2-yl)methyl)sulfinyl) ethyl thioformate compound, a preparation method of the compound and a process for preparing esomeprazole by using the compound. The compound and the preparation method have the remarkable advantages that: raw materials for synthesis are cheap and can be easily obtained; the reaction conditions are moderate; the yield of the prepared esomeprazole is high; the optical purity is high; the operation is safe; the environment is slightly polluted; and the compound is more suitable for industrial large-scale production.
Owner:CHENGDU EASTON BIOPHARMACEUTICALS CO LTD
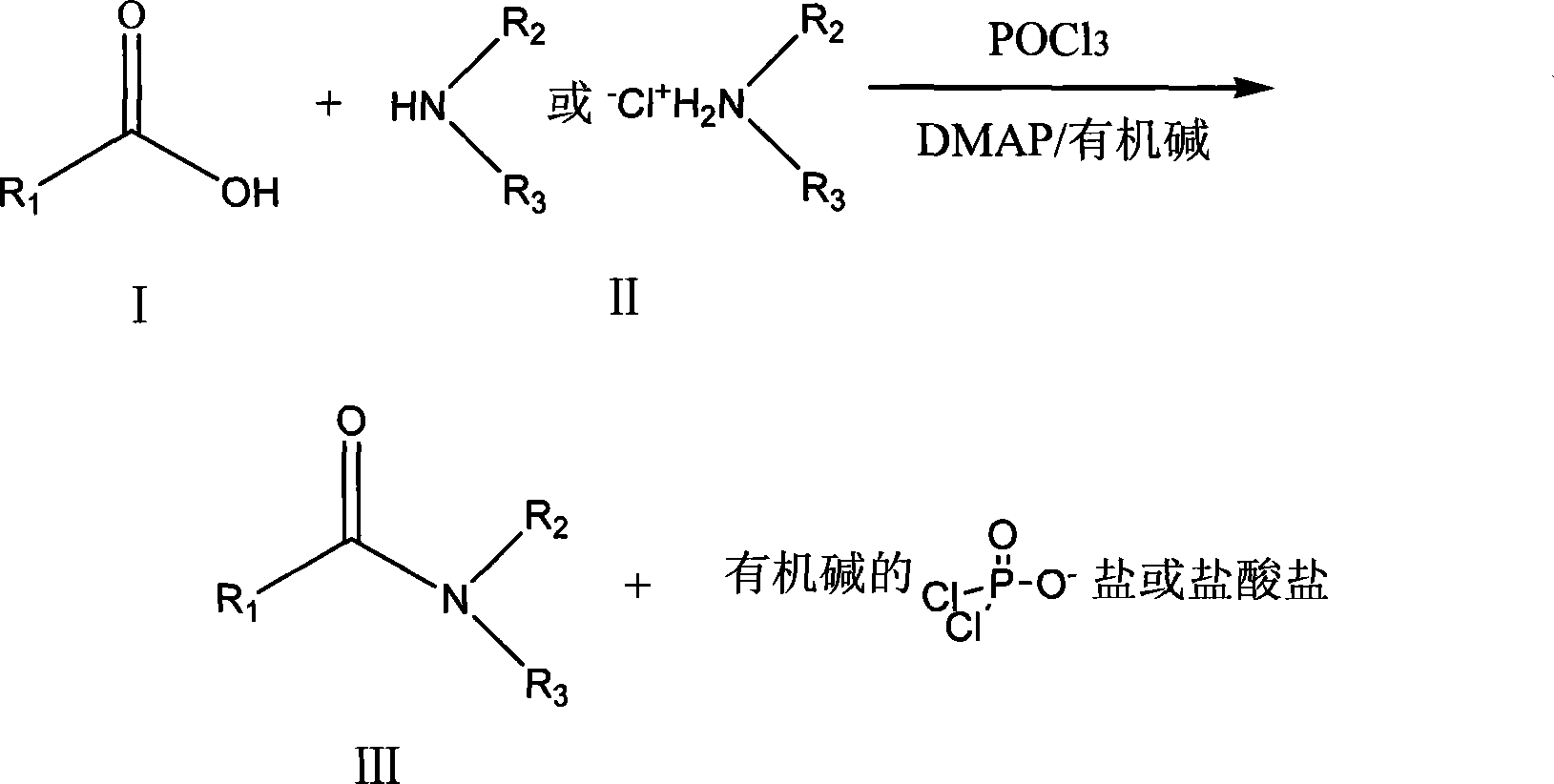
Method for generating amido bond
InactiveCN101235078ALow priceEasy to getOrganic compound preparationCarboxylic acid amides preparationOrganic solventCarboxylic acid
A generation method of amido bond relates to a method for forming amido bond, which comprises in the presence of phosphoryl halide, N, N-dimethylpyridine (DMAP) and organic alkali, reacting dimethylpyridine and free amine or amine salt in organic solvent to generate amido bond, with low cost, simple process, high yield, non racemic generation, simple operation and support for industrial production. The invention comprises dissolving carboxyl acid and free amine or carboxyl acid and amine salt into organic solvent to be reacted, adding organic alkali and catalyst N, N-dimethylpyridine, adding phosphoryl halide, generating amido bond between the carboxy group of carboxyl acid and amido of amine to obtain the slat and hydrochlorate of relative amide and organic alkali.
Owner:XIAMEN UNIV
Method for preparing 2,3,5-trimethylpyridine
The invention discloses a making method of 2, 3, 5-trimethyl pyridine, which comprises the following steps: 1) making composite catalyst of cobalt aluminium phosphate with acid degree adjuster; 2) placing the composite catalyst into fixed bed reactor; aerating propanal and ammonia gas; looping to generate 2-ethyl-3, 5-dimethyl pyridine; 3) demethylating 2-ethyl-3, 5-dimethyl pyridine and sulfur flour; obtaining the product.
Owner:ZHEJIANG UNIV +1
Method for synthesizing pyridinecarbaldehydes compound with direct oxidization method
InactiveCN101898999ABroaden applicationApplicable and reliableOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsCatalytic oxidation
The invention refers to a method for synthesizing pyridinecarbaldehydes compound with direct oxidization method, comprising the following specific steps: synthesizing catalyst, and selectively catalyzing and oxidizing methyl pyridine, by using catalytic synthesis technology and synthesis method of catalyst that a series of methyl pyridines at different substitutional positions are directly oxidized to synthesize pyridinecarbaldehyde; by using homogeneous phase complexing catalytic process, directly catalyzing and oxidizing material methyl pyridine or dimethyl pyridine at normal pressure to synthesize corresponding methyl substituted pyridinecarbaldehydes product with high catalytic efficiency, product conversion rate and product yield; the reaction conditions are simple and convenient; and the method is suitable for industrial production in large scale.
Owner:EAST CHINA UNIV OF SCI & TECH
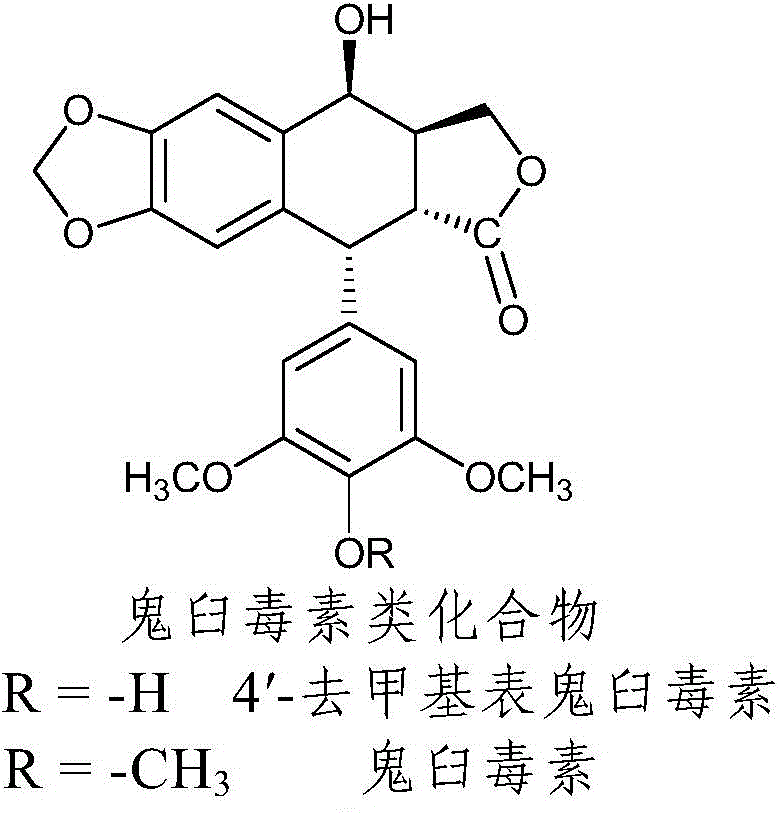
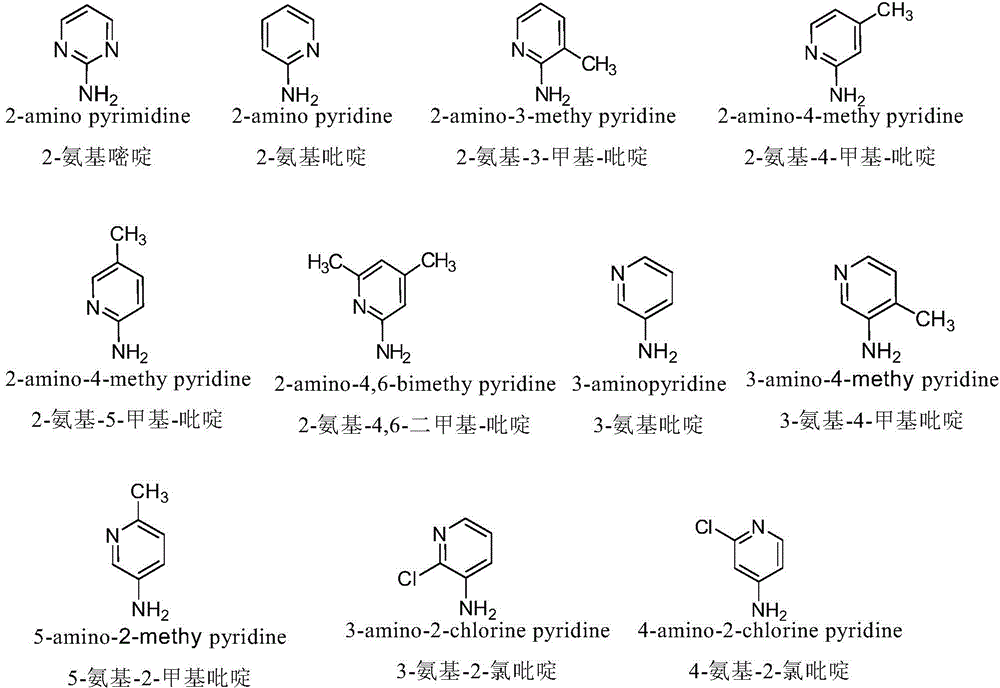
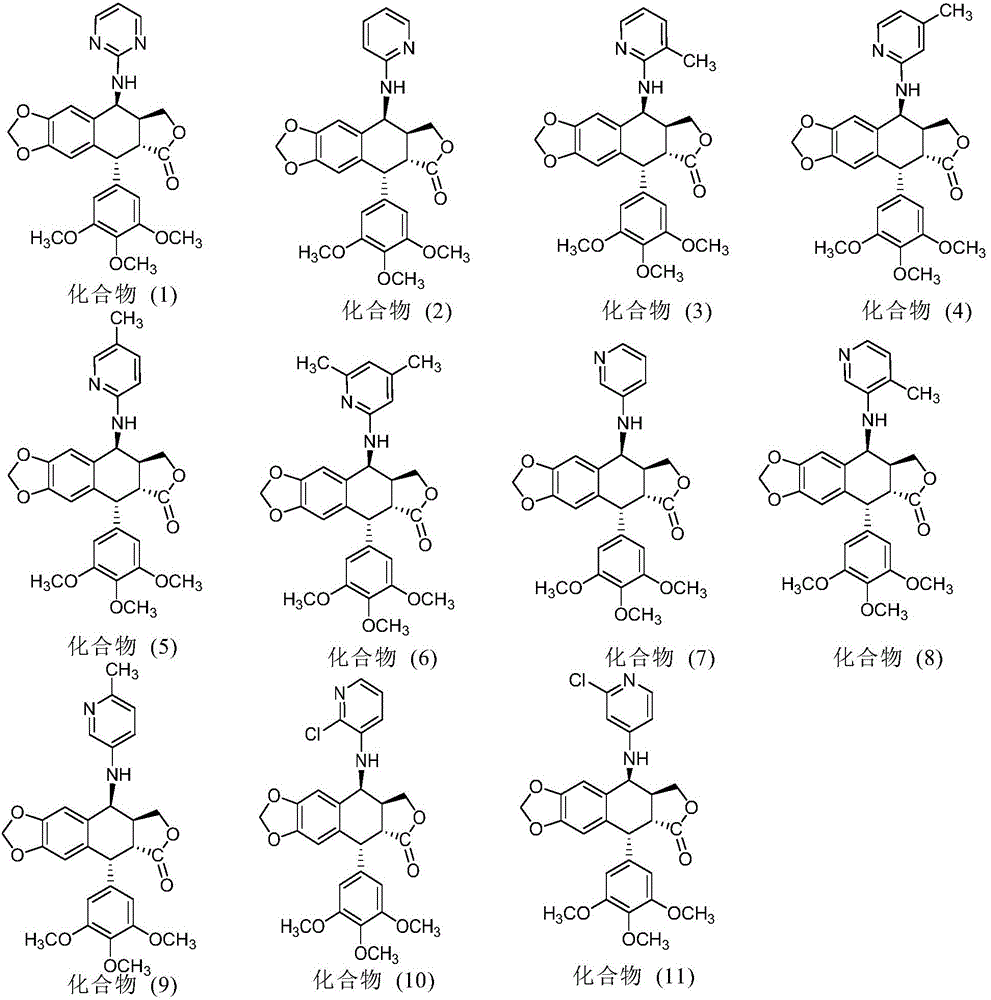
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
PH fluorescent probe and application of same
InactiveCN105131937ALarge Stokes shiftUnique Fluorescence SelectivityOrganic chemistryFluorescence/phosphorescenceRefluxPyridinium
The invention discloses a pH fluorescent probe chemically named iodized-1-methyl-2-(4-hydroxy-3-methoxy)styryl pyridinium salt. A synthetic method includes the following steps: adding iodized-1,2-dimethyl pyridinium salt and vanillic aldehyde into a n-butyl alcohol solution according to the molar ratio of 1:1-1:3, adding piperazine and performing a reaction under reflux for 0.5-1 h to obtain the iodized-1-methyl-2-(4-hydroxy-3-methoxy)styryl pyridinium salt, wherein the mass volume ratio of the vanillic aldehyde to the n-butyl alcohol solution is 0.304:20-0.456:25; and the mass ratio of the vanillic aldehyde to the piperazine is 0.456:0.02-0.316:0.01. The compound is very obvious in solution color change under different pH values, wherein the color is converted into red from green, so that the pH value of environment can be determined according to the color change of the solution. The compound is an excellent visual pH fluorescent probe.
Owner:TAISHAN MEDICAL UNIV
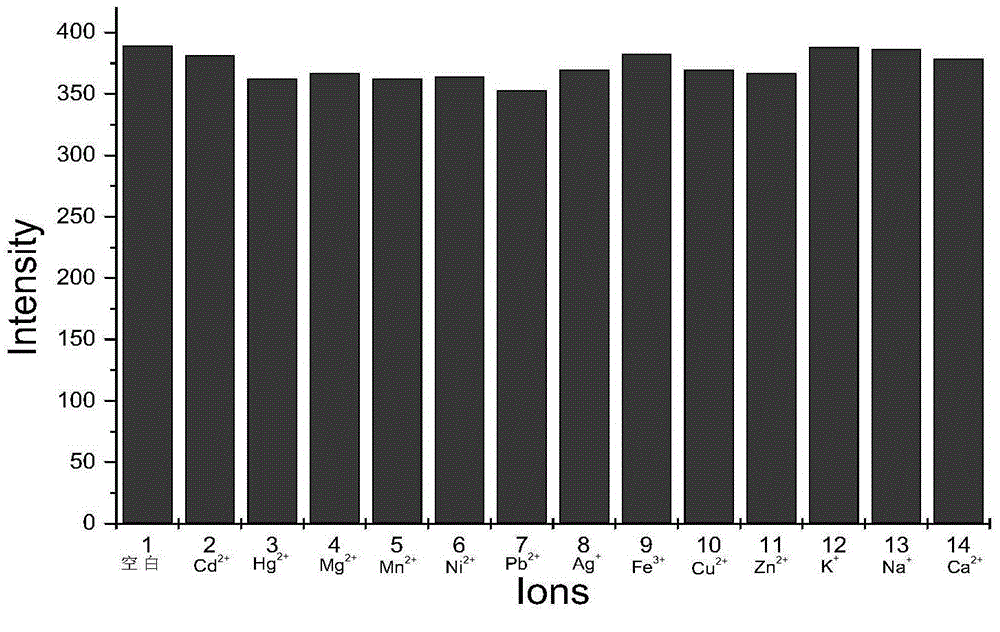
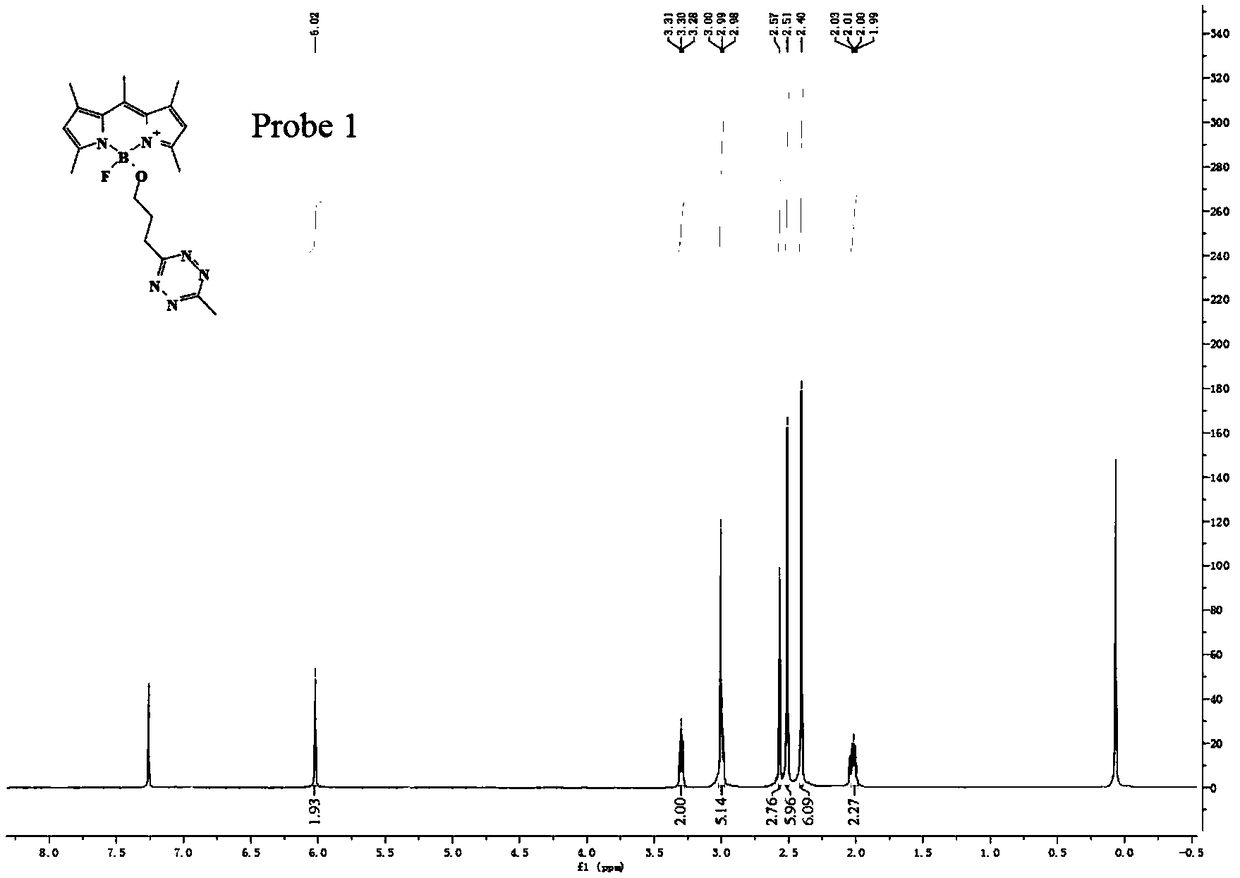
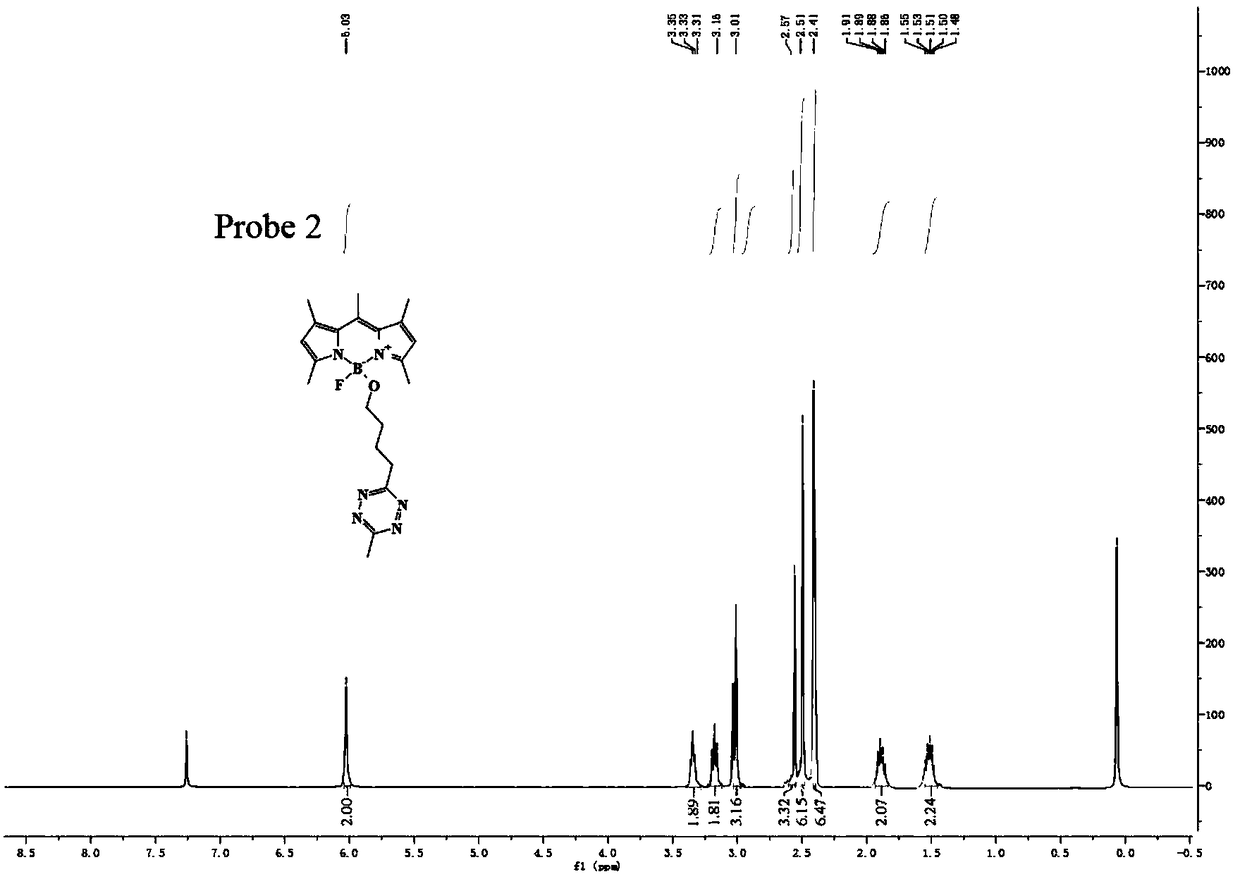
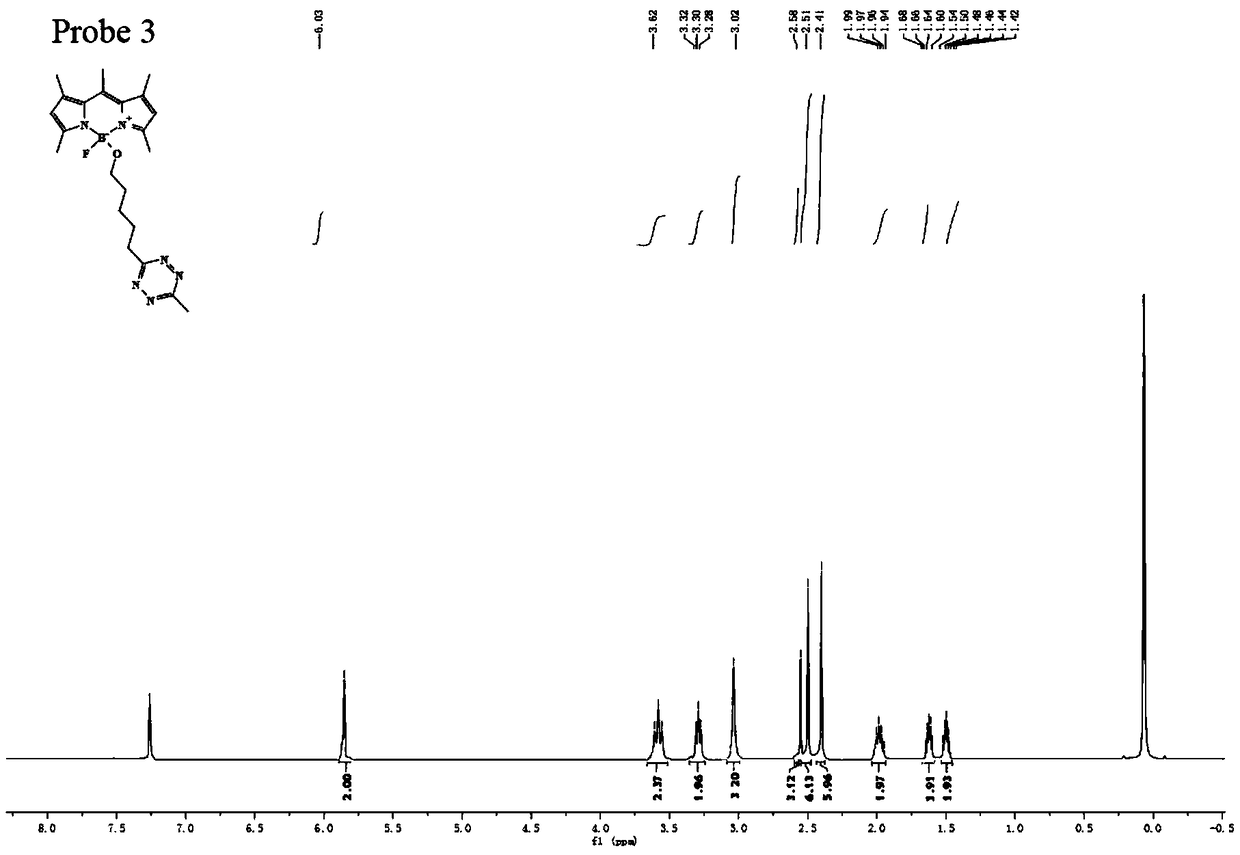
Preparation method of BODIPY-tetrazine bio-orthogonal probe
InactiveCN108997402AHigh priceSolve complexityGroup 3/13 element organic compoundsFluorescence/phosphorescenceSolubilityOrganic layer
The invention discloses a BODIPY-tetrazine bio-orthogonal probe and a preparation method thereof. The preparation method comprises the steps: 1) dissolving BODIPY1-2 and TMSOTf in an anhydrous acetonitrile-dichloromethane mixed solvent, mixing evenly, stirring the reaction solution for 30 min under a condition of the temperature of 0 DEG C, and activating BODIPY1-2; 2) adding tert-butanol quenchedTMSOTf to the reaction solution, then dropwise adding 2,6-dimethylpyridine and a tetrazine Tza-Tzg pre-mixed acetonitrile solution, and carrying out a stirring reaction of the mixed solution under acondition of the temperature of 0 DEG C for 10 min; and 3) quenching the reaction in the step 2 by water, then extracting by DCM, separating an organic layer, drying the organic phase by Na2SO4, concentrating in vacuum, carrying out separated purification of the reaction product by a silica gel column, and thus obtaining the BODIPY-tetrazine bio-orthogonal fluorescent probe. The BODIPY-tetrazine bio-orthogonal probe is synthesized by the reaction of the BODIPY compound with the tetrazines. The yield in the synthesis process is greatly increased, the water solubility of the probe is greatly improved, the probe has the fluorescent opening properties, and the improvements are conducive to an application of the probe in cell imaging.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00001.png)
![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00002.png)
![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00003.png)


![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/DSA00000351862100011.png)
![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/FSA00000351862200011.png)
![Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide Preparation method of 1-(2,2-difluoroethoxy)-6-trifluoromethyl-N-([1,2,4]triazolezol[1,5-C] pyrimidine-2-)benzsulfamide](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/513cbca7-c8d0-42d7-a898-6e6990555546/FSA00000351862200021.png)