Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
166 results about "3-Methylpyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
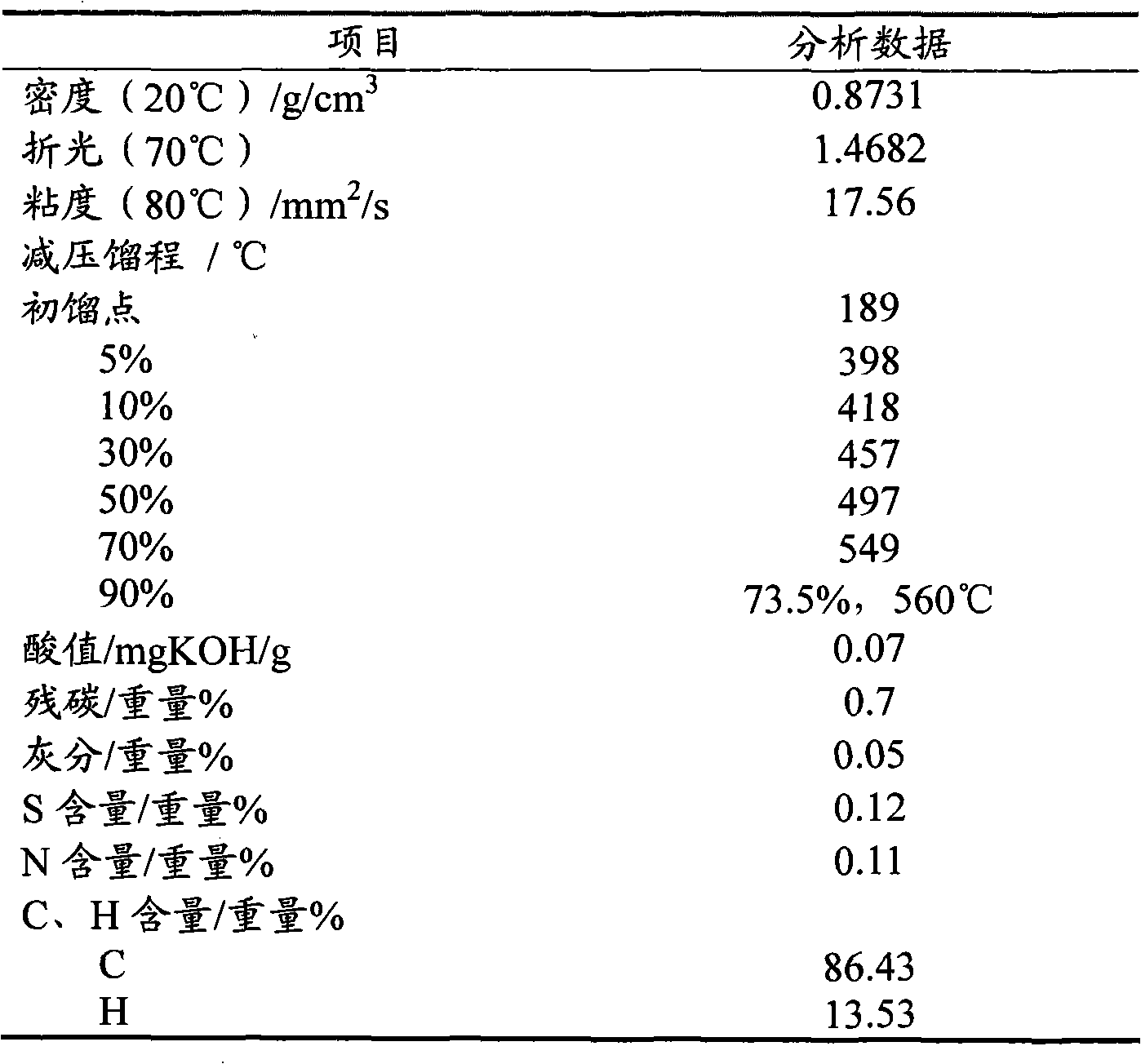
3-Methylpyridine or 3-picoline, is an organic compound with formula 3-CH₃C₅H₄N. It is one of three positional isomers of methylpyridine, whose structures vary according to where the methyl group is attached around the pyridine ring. This colorless liquid is a precursor to pyridine derivatives that have applications in the pharmaceutical and agricultural industries. Like pyridine, 3-methylpyridine is a colorless liquid with a strong odor and is classified as a weak base.
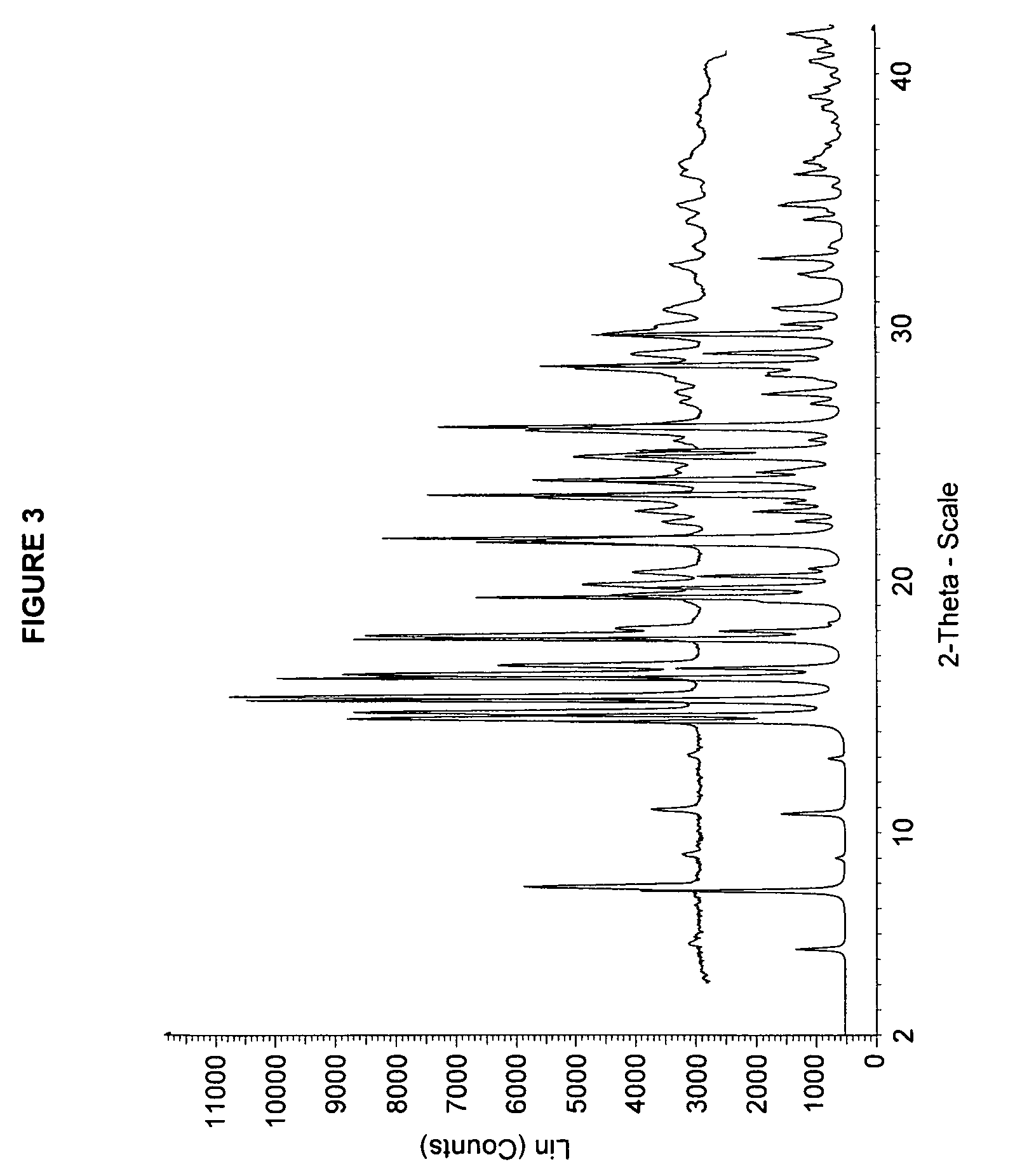
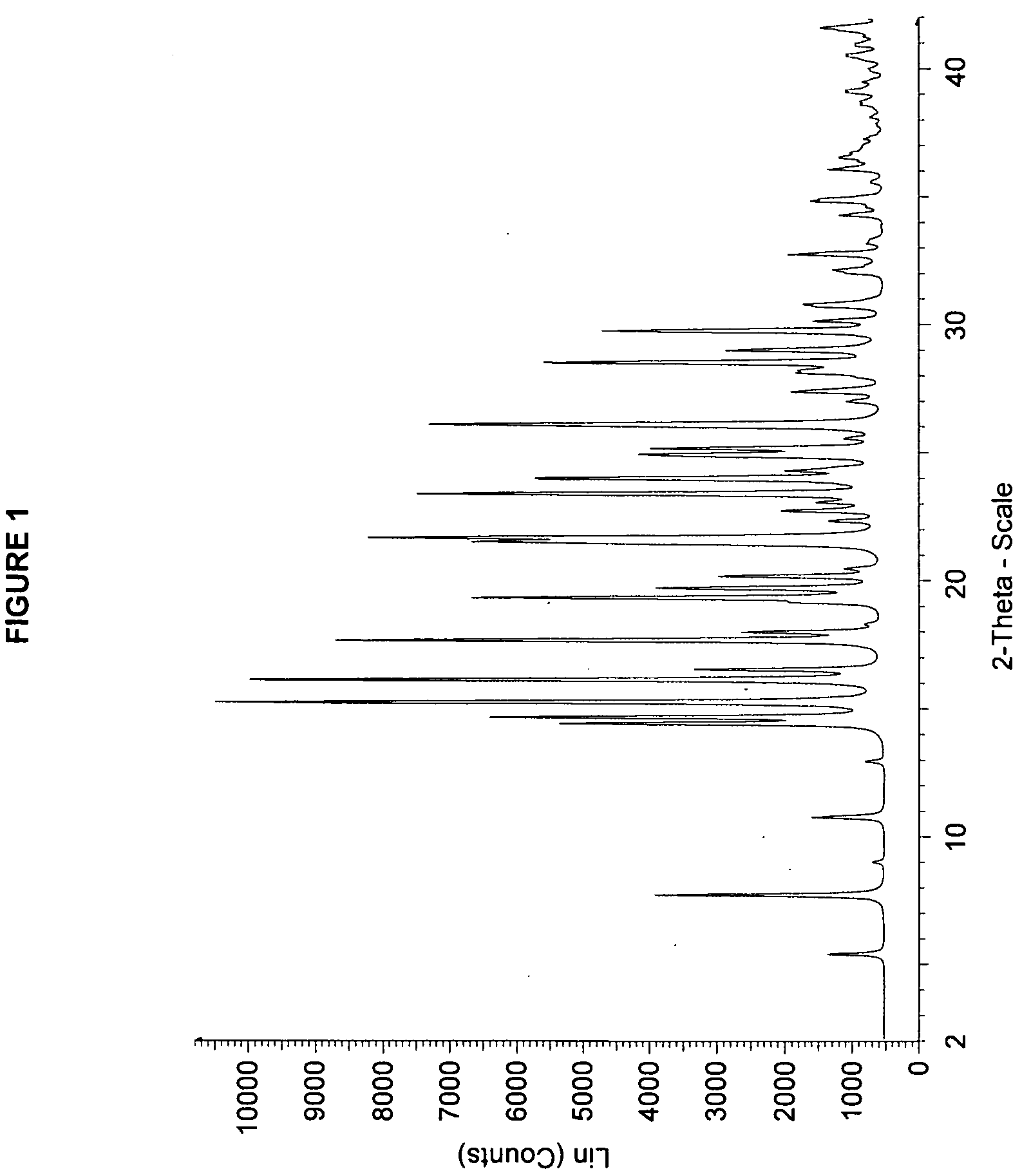
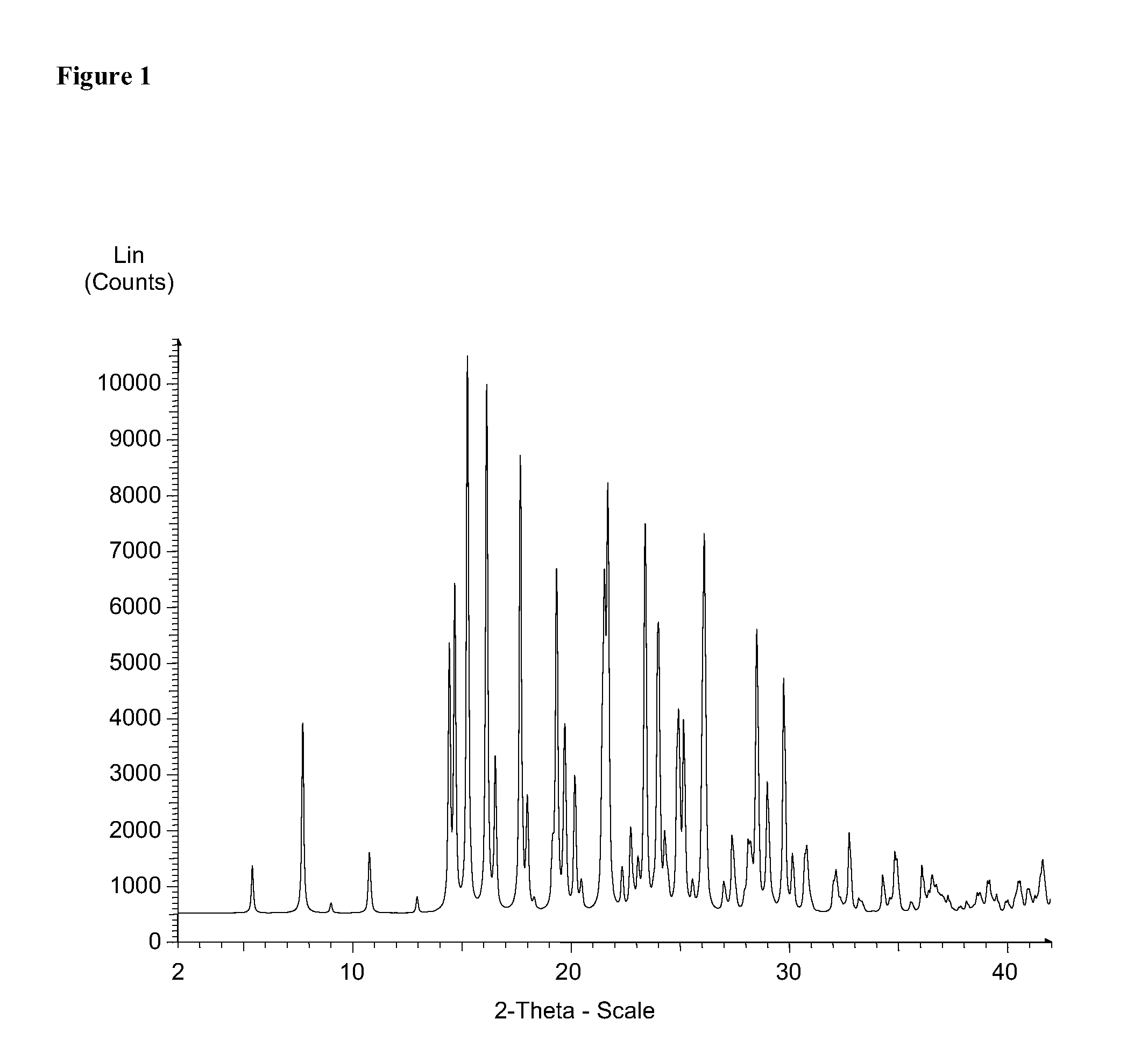
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
The present invention relates to a substantially crystalline and free solid state form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Form I), pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Processes for producing cycloalkylcarboxamido-pyridine benzoic acids
The present invention relates to a process of providing the 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid in substantially free form (Compound 1).
Owner:VERTEX PHARMA INC
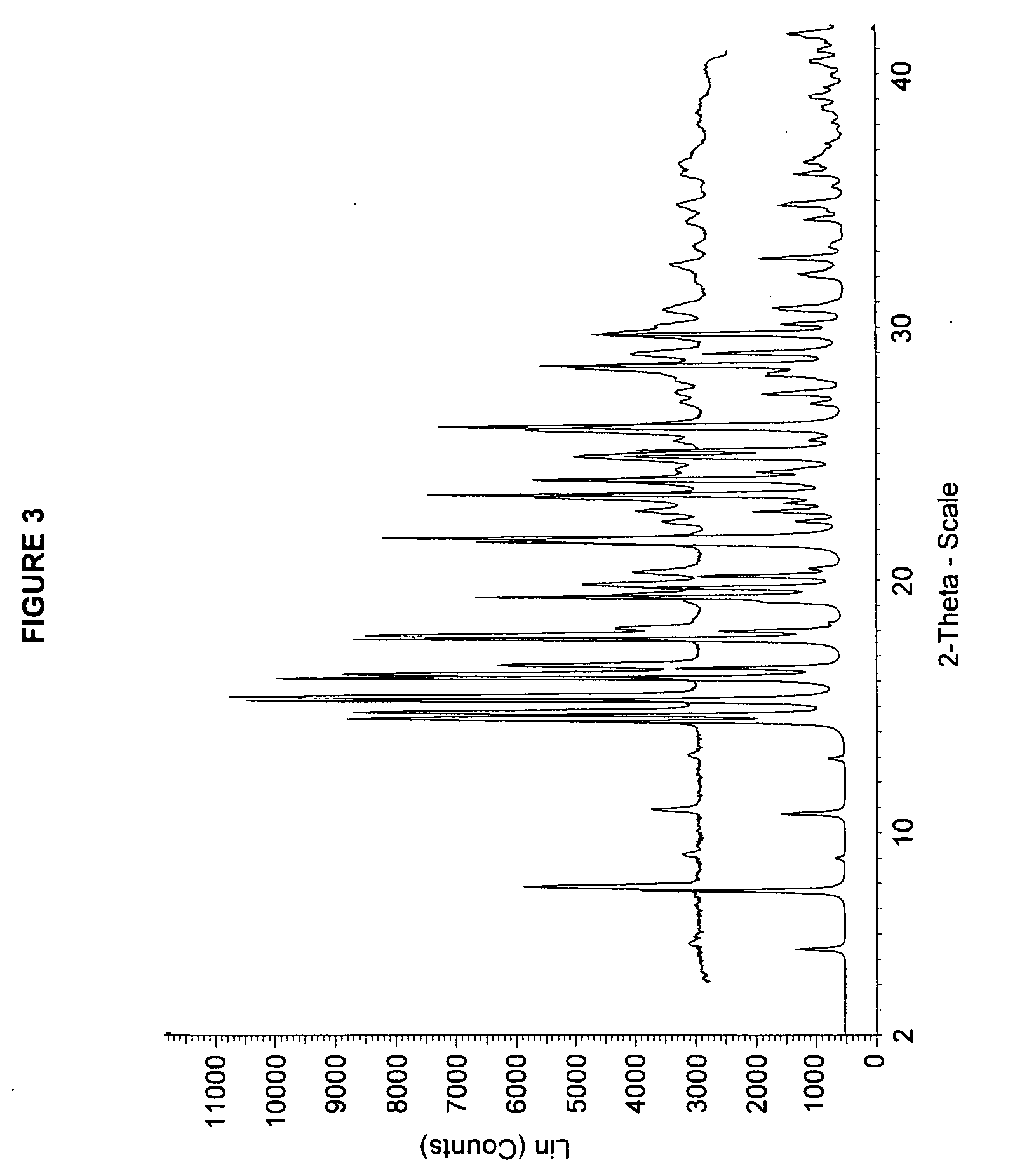
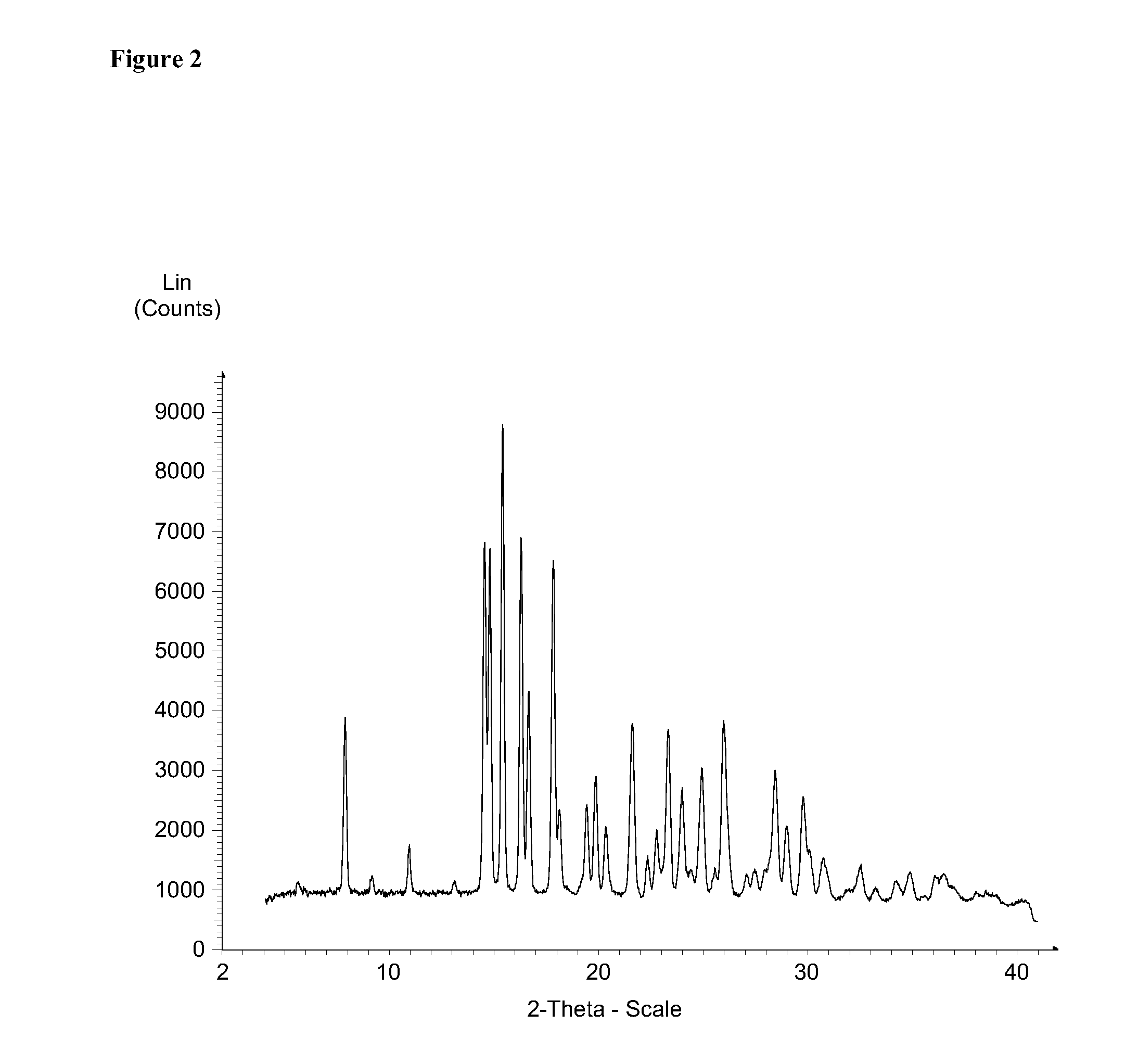
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
InactiveUS20130085158A1Affect characteristicReduce severityBiocideSenses disorderBenzoic acidMedicinal chemistry
The present invention relates to a substantially a solid form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Compound 1, Solvate Form A and Compound 1, HCl Salt Form A), processes for making such forms, pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
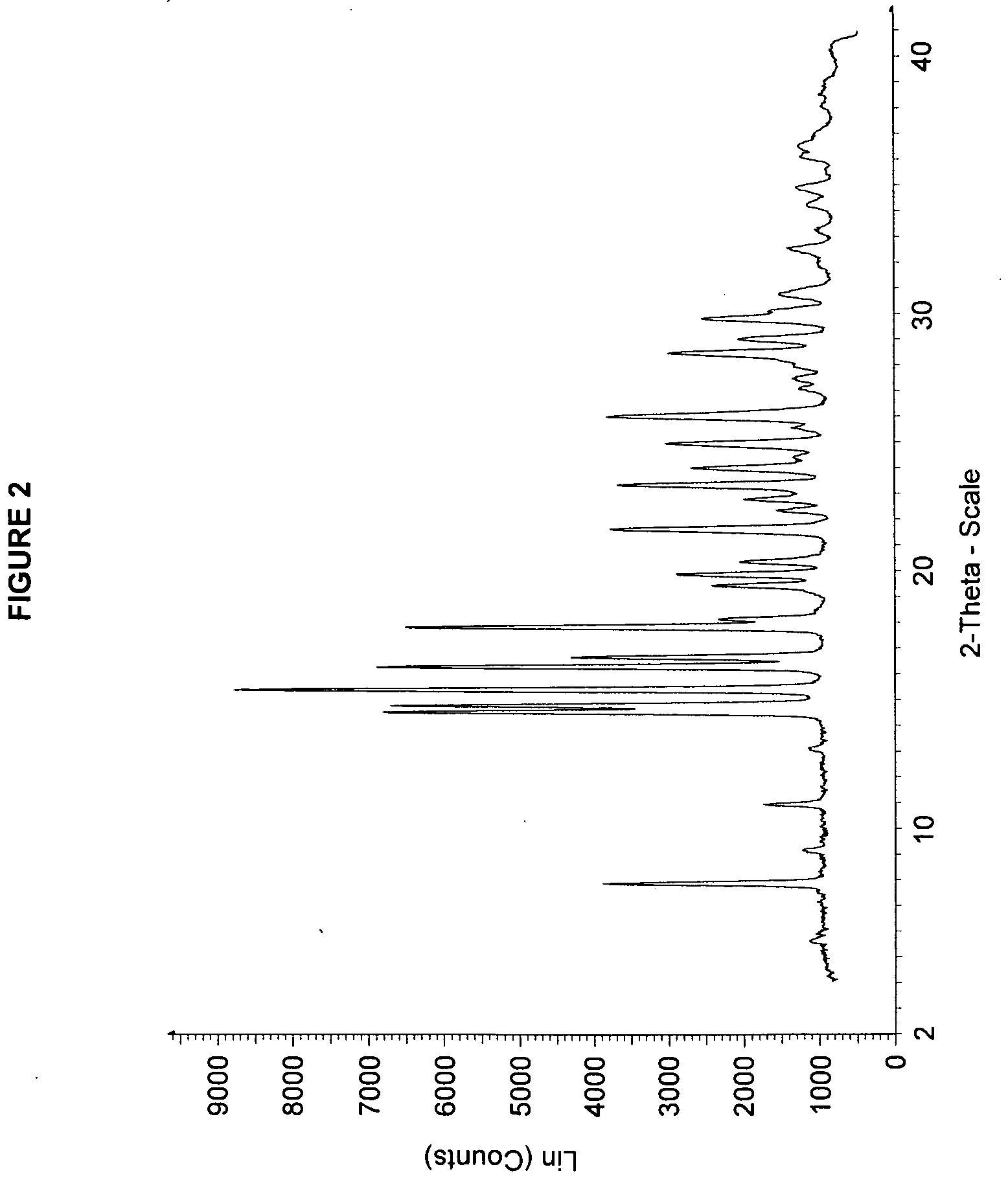
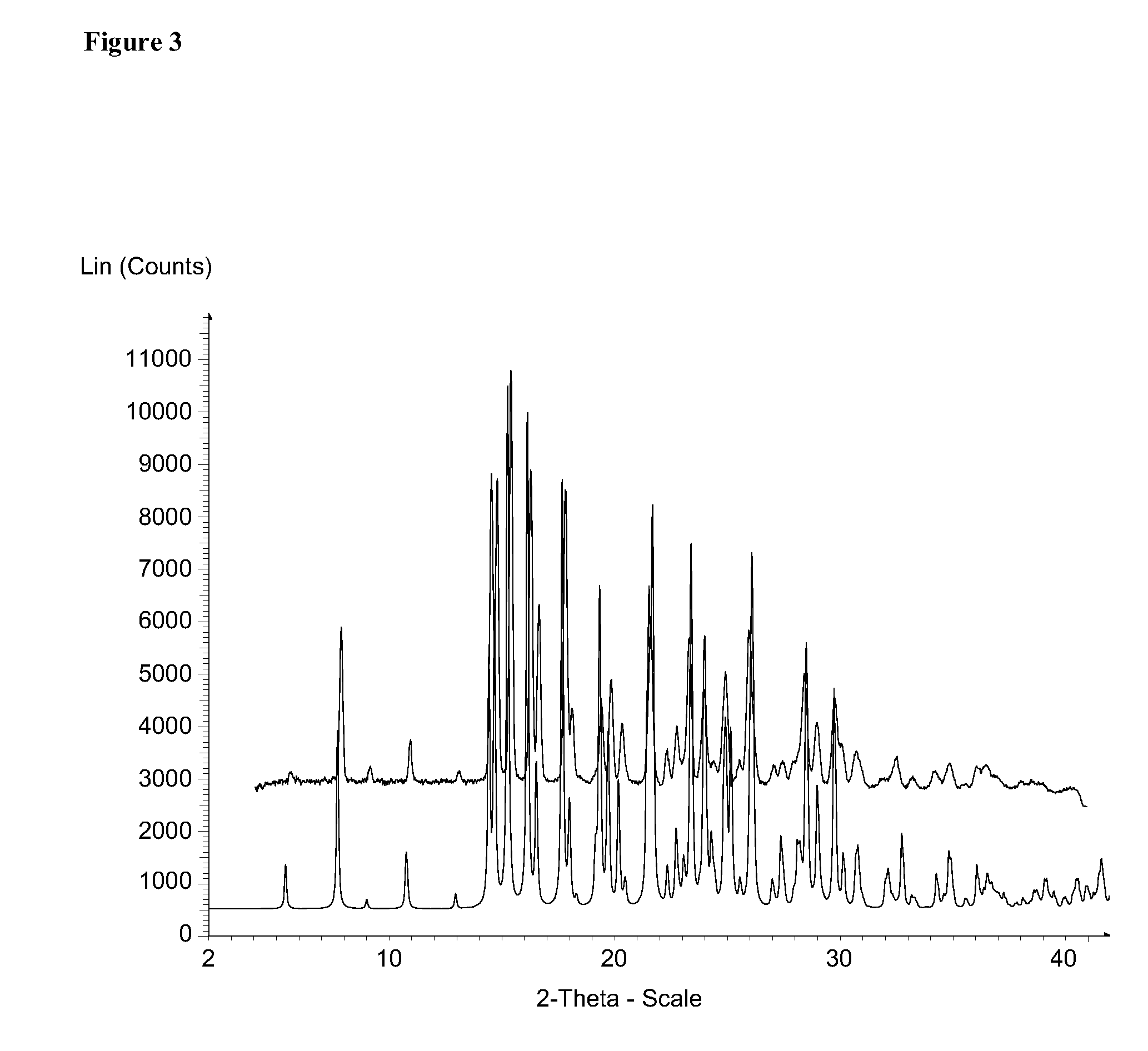
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
ActiveUS20110263654A1Reduce severityAffect characteristicBiocideSenses disorderBenzoic acidMedicinal chemistry
The present invention relates to a substantially a solid form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Compound 1, Solvate Form A and Compound 1, HCl Salt Form A), processes for making such forms, pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
The present invention relates to formulations of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid in Form I, pharmaceutical packs or kits thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Processes for producing cycloalkylcarboxamido-pyridine benzoic acids
ActiveUS20090176989A1Useful in treatmentTreating and lessening severityOrganic active ingredientsOrganic chemistryBenzoic acidFree form
The present invention relates to a process of providing the 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid in substantially free form (Compound 1).
Owner:VERTEX PHARMA INC
Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
The present invention relates to a substantially crystalline and free solid state form of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid (Form I), pharmaceutical compositions thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Processes for producing cycloalkylcarboxamido-pyridine benzoic acids
The present invention relates to a process of providing the 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid in substantially free form (Compound 1).
Owner:VERTEX PHARMA INC
Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid
ActiveUS20140206720A1Treating and lessening severityBiocideOrganic chemistryBenzoic acidPharmacology
The present invention relates to formulations of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid in Form I, pharmaceutical packs or kits thereof, and methods of treatment therewith.
Owner:VERTEX PHARMA INC
Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides
ActiveUS20050215570A1Easy to optimizeQuick responseBiocideOrganic active ingredientsArylSulfonyl chloride
Owner:CORTEVA AGRISCIENCE LLC
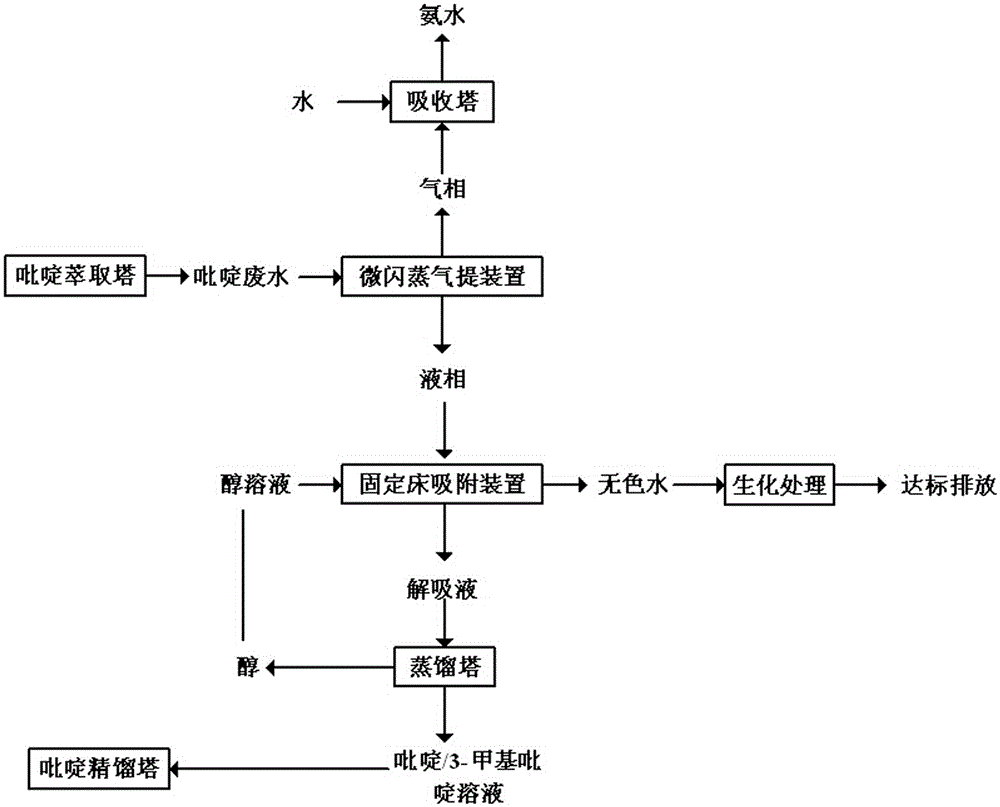
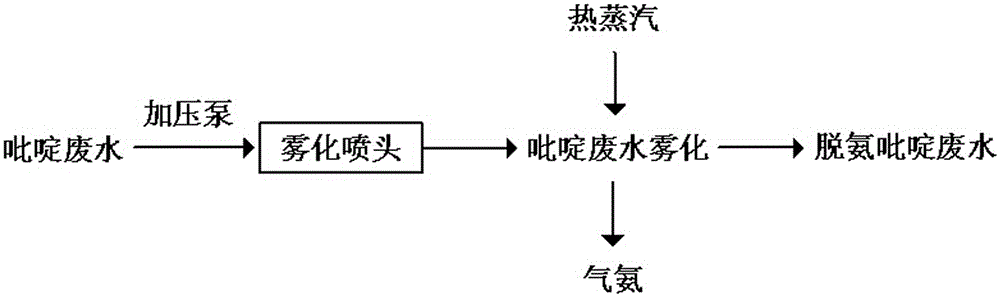
Method for treating pyridine wastewater
ActiveCN105084671AReduce processing costsOrganic chemistryMultistage water/sewage treatmentDesorptionDistillation
The invention discloses a method for treating pyridine wastewater, which comprises the following steps: pyridine wastewater, which is discharged from a pyridine extraction tower in an acetaldehyde+formaldehyde+liquid ammonia high-temperature pyridine synthesis technique, is subjected to low-vacuum flash distillation gas stripping, gas-phase absorption, fixed bed adsorption, desorption, distillation and other steps to separate and recover ammonia, pyridine and 3-methylpyridine in the pyridine wastewater, and the colorless water is subjected to biochemical treatment and discharged after reaching the standard. The method solves the problems of discharge of incinerator exhaust NOX, high incineration cost and carbon discharge due to abundant pyridine and derivatives in the wastewater incineration process in the pyridine production technique, effectively recovers the pyridine and derivatives, saves the cost, and lowers the pollution.
Owner:河北美邦工程科技股份有限公司
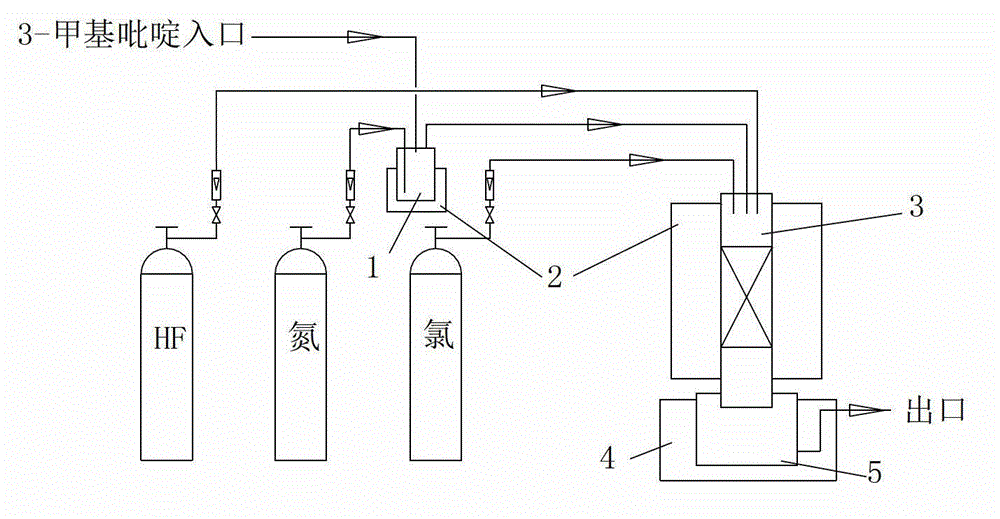
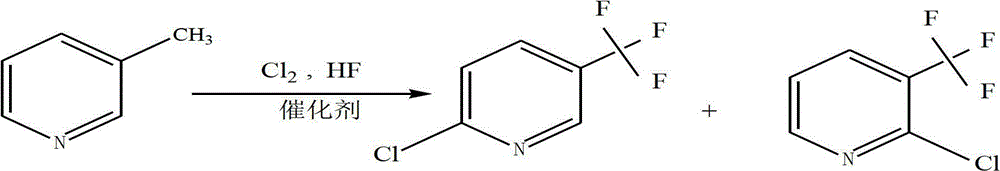
2-chloro-5-tirfluoromethylpyridine and synthetic method thereof
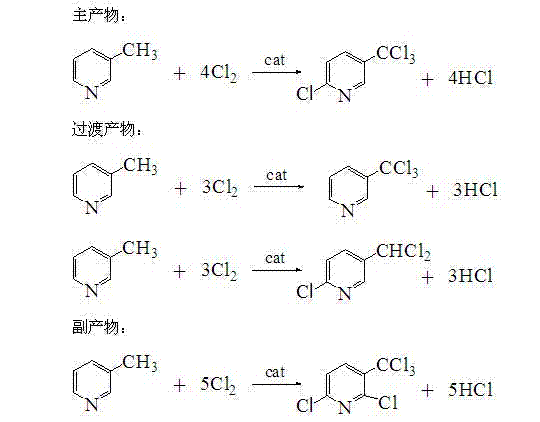
The invention discloses a 2-chloro-5-tirfluoromethylpyridine and a synthetic method thereof. The synthetic method of the 2-chloro-5-trifluoromethylpyridine comprises the following steps of: (1) feeding 3-methylpyridine into a vaporizer to vaporize the 3-methylpyridine; (2) adding a catalyst into a reactor and heating the reactor to a reaction temperature, and feeding chlorine gas and hydrogen fluoride gas into the reactor; and (3) by utilizing nitrogen as a carrier gas, carrying the vaporized 3-methylpyridine into the reactor so as to be subjected to reaction, and condensing, purifying and separating the gas after reaction, so that 2-chloro-5-trifluoromethylpyridine and 2-chloro-3-trifluoromethylpyridine are obtained. According to the synthetic method disclosed by the invention, the cobalt dichloride catalyst is available and is lower in price, industrialization is easy to realize, catalytic selectivity to two target products is high, and the yield is high; and nitrogen is adopted as the carrier gas, and the nitrogen is available, low in cost and safer and has less possibility of influencing the environment, so that the synthetic method disclosed by the invention is beneficial to industrial production.
Owner:潍坊新绿化工有限公司
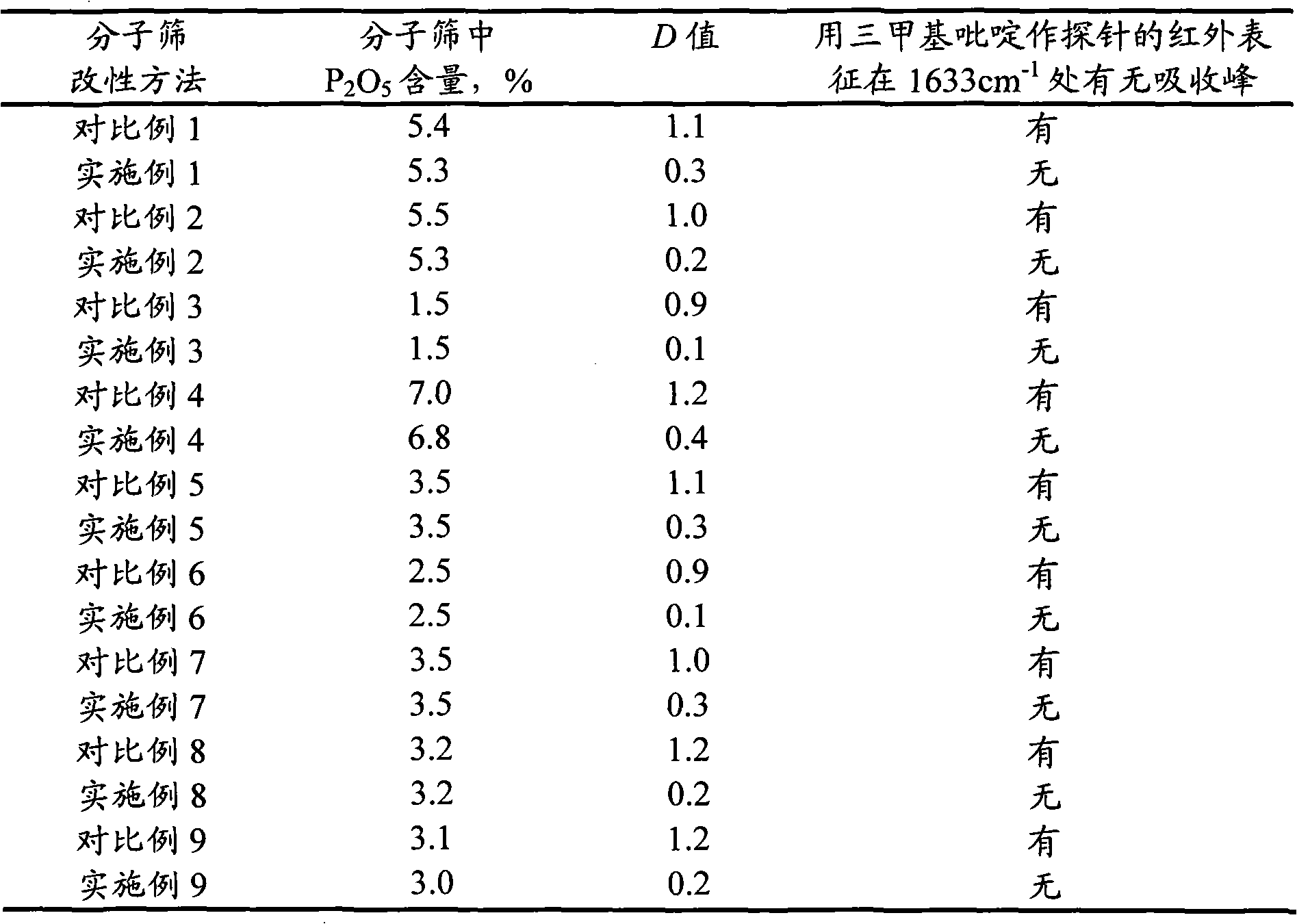
MFI structure molecular sieve containing phosphorus and transition metals, and preparation method thereof
ActiveCN102838130AReduce yieldHigh yieldMolecular sieve catalystsMolecular-sieve and base-exchange phosphatesMolecular sieveHydrogen
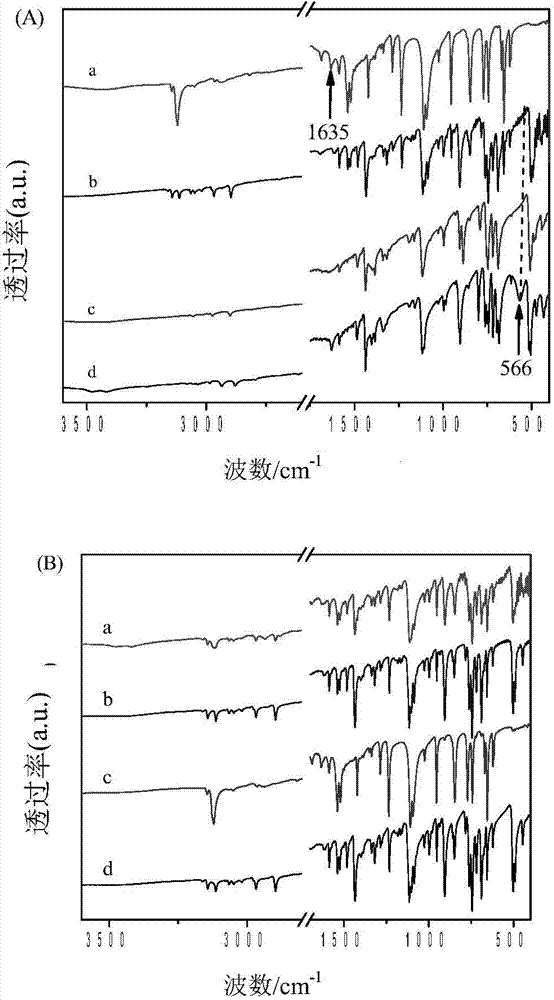
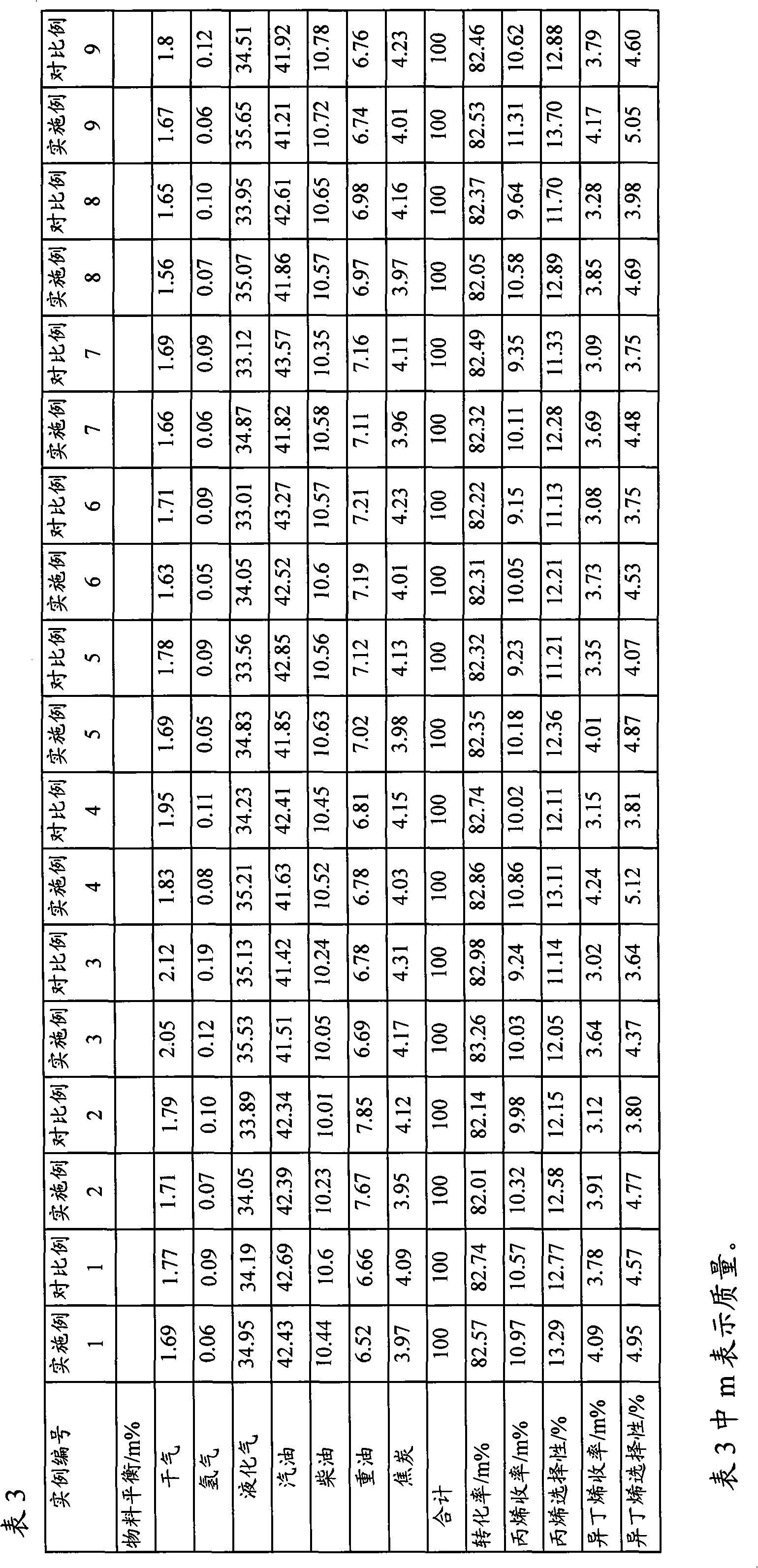
The invention relates to a MFI structure molecular sieve containing phosphorus and transition metals, and a preparation method thereof. According to the MFI structure molecular sieve of the present invention, phosphorus distribution D meets the following conditions that D is more than or equal to 0 and less than or equal to 0.8, and is equal to P(S) / P(C), wherein P(S) represents the phosphorus content in a part from the edge to 1 / 5 of the center of the molecular sieve grain characterized by using a TEM-EDX method, P(C) represents the phosphorus content in the center of the molecular sieve grain, and no absorption peak exists at 1633 cm<-1> on an infrared spectrogram obtained by adopting 3-methylpyridine as a probe. The preparation method for the molecular sieve comprises: mixing the calcined MFI structure molecular sieve containing phosphorus and transition metals with a silicon source, carrying out reaction crystallization for 2-80 hours at a temperature of 145-190 DEG C, carrying out separating and drying, and carrying out calcination at a temperature of 400-800 DEG C. The molecular sieve of the present invention is used for catalytic cracking, wherein light olefin yield is high, and yields of coke, hydrogen and dry gas are reduced.
Owner:CHINA PETROLEUM & CHEM CORP
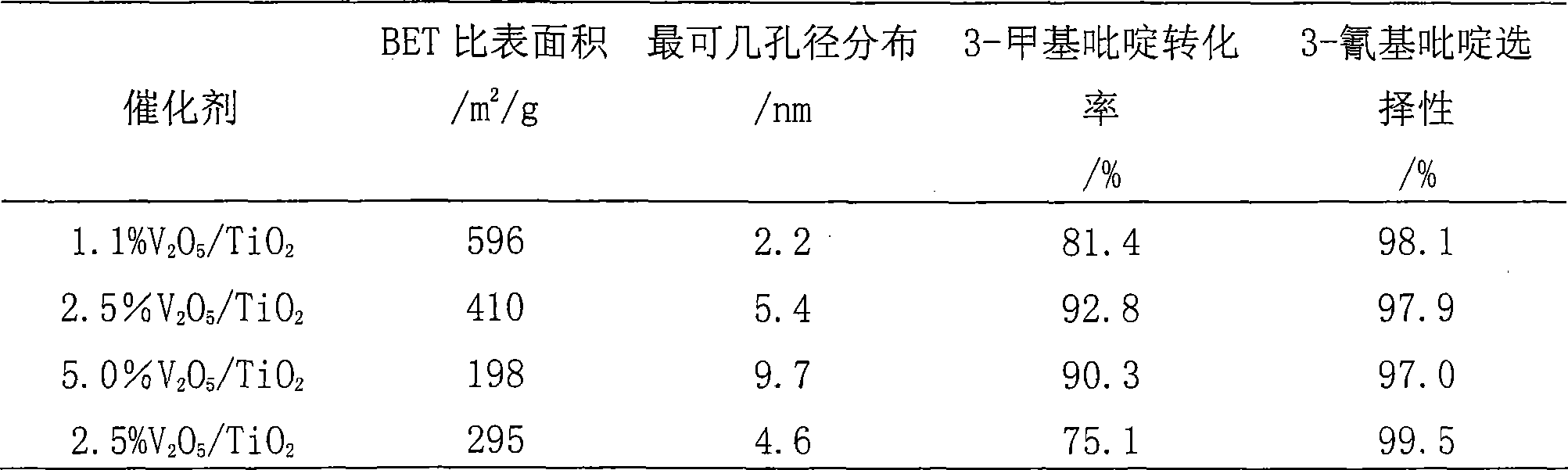
Mesoporous TiO2 supported V2O5 catalyst with high specific surface area as well as preparation method and use thereof
InactiveCN101433835ANot deactivatedNo apparent inactivationOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsGas phaseReaction temperature
The invention relates to a mesoporous TiO2 supported V2O5 catalyst with high specific surface area and a preparation method and application thereof. Aiming at the disadvantages that the prior V2O5Tio2 has low catalytic activity, high reaction temperature, and poor selectivity and catalyst stability when 3-methylpyridine is ammoxidized to produce 3-cyanopyridine, the invention provides a mesoporous TiO2 supported V2O5 catalyst which has low reaction temperature, high transformation rate and good selectivity, is used for producing the 3-cyanopyridine by ammoxidizing the 3-methylpyridine and has high specific surface area, and a preparation method and application thereof. The catalyst comprises a carrier and an active constituent, the carrier adopts TiO2, and the active constituent adopts V2O5. The method for preparing the catalyst comprises the following steps: a template agent adopts organic amine, a titanium source adopts tetraisopropoxy titanium, a vanadium source adopts triisopropoxyvanadium oxide, and the TiO2 supported V2O5 catalyst is directly synthesized in a polytetrafluorine kettle under hydrothermal conditions. The catalyst is used for producing the 3-cyanopyridine by ammoxidizing the 3-methylpyridine in a gas phase.
Owner:ZHEJIANG NORMAL UNIVERSITY
Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors
ActiveCN102260266AHas inhibitory activityOrganic active ingredientsNervous disorderPhosphodiesterasePhenyl group
The invention discloses pyrazolo[3,4-d]pyrimidone compounds and application thereof in preparation of a phosphodiesterase IX inhibitor. The structure of the compounds is shown as a formula (1); in the formula, when R' is 2-chlorophenyl, R refers to phenyl, substituted phenyl, benzyl, substituted benzyl, 3-methylpyridine, 1-phenylethyl, diphenylmethyl or CHCH3CONHR1, wherein R1 refers to phenyl ormethyl, methoxyl, ethoxyl, isopropoxy, methylthio, NHCOCH3 or NCH3CH3 substituted phenyl; when R' is phenyl, R refers to CHCH3CONHR2, wherein R2 refers to methoxyl or ethoxyl substituted phenyl; and when R' is methyl, R refers to 4-methoxy-benzyl, diphenylmethyl or N-(2-amino-cyclohexyl)-4-methoxy-benzenesulfonamide. The pyrazolo[3,4-d]pyrimidone compounds have activity of inhibiting phosphodiesterase IX, can serve as the phosphodiesterase IX inhibitor, can be used for preparing the phosphodiesterase IX inhibitor, and have wide application prospect. The compounds are shown as the formula (1).
Owner:SUN YAT SEN UNIV
Preparation method of pyridine and 3-picoline
The invention relates to a preparation method of pyridine and 3-picoline by loading ZSM-5 zeolite with metallic oxides selected form cobalt, lead or its mixture as catalyst, reacting the mixture of formaldehyde and acetaldehyde with ammonia in gaseous phase, thus obtaining high yield of pyridine and 3-methyl pyridine.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for preparing 2-chloro-5-trichloromethylpyridine
InactiveCN102452977AEasy to control ratioProcess conditions are easy to controlOrganic chemistryInorganic compoundReaction temperature
The invention relates to a method for preparing 2-chloro-5-trichloromethylpyridine, which is characterized by comprising the following steps of: initiating reaction of 3-methylpyridine serving as a raw material in the presence of an organic solvent and chlorine gas by a one-step liquid-phase chlorination method by using an initiator to synthesize the 2-chloro-5-trichloromethylpyridine, and purifying to obtain a finished product, wherein the reaction temperature is 100 to 160 DEG C, reaction time is 15 to 17 hours, and the initiator is formed by combining an organic compound initiator with an inorganic compound initiator and is added in an amount which is 3 to 6 percent based on the weight of the 3-methylpyridine serving as the raw material. The liquid-phase chlorination method is adopted, so that the raw material reacts in a liquid state, the ratio of reactants and reaction conditions are easy to control, and byproducts are reduced; and the inorganic initiator and the organic initiator are used simultaneously, so that the yield of the target product reaches 84 percent, and the method is an ideal method for synthesizing the 2-chloro-5-trichloromethylpyridine.
Owner:NINGBO UNIV
Preparation method of 2-chloro-5-trichloromethylpyridine
InactiveCN104496890AFully contactedIncrease the heat exchange areaOrganic chemistryOrganic solventChemical combination
The invention discloses a preparation method of 2-chloro-5-trichloromethylpyridine, and belongs to the technical field of chemical combination. The method comprises the following steps: by adopting 3-methylpyridine as a raw material, under the condition of presence of an organic solvent and an initiator, carrying out one-step liquid-phase chlorination on a chlorine gas and 3-methylpyridine in a micro-reactor, so as to synthesize 2-chloro-5-trichloromethylpyridine; firstly, desolventizing, and separating and purifying by a high-vacuum distillation method; separating and recycling a chlorinated transitional product to further react with the raw material; and purifying, so as to obtain the 2-chloro-5-trichloromethylpyridine product. The preparation method of 2-chloro-5-trichloromethylpyridine is full in gas-liquid contact, large in heat exchange area, and mild in reaction condition; the side reaction can be reduced; and the yield can reach 72%-80%.
Owner:JIANGSU YANGNONG CHEM GROUP
Production method of high-purity 2-chloro-5-trifluoromethylpyridine
The invention provides a production method of high-purity 2-chloro-5-trifluoromethylpyridine. The production method comprises the following steps: under the action of a silicon dioxide-aluminum oxide-chromic oxide catalyst, gasifying the raw materials acrolein and propyl aldehyde, and reacting with an ammonia gas to generate 3-methylpyridine; gasifying the 3-methylpyridine and carbon tetrachloridetogether, reacting in a chlorine atmosphere to obtain 2-chloro-5-trichloromethylpyridine, and performing fluoro-substitution to obtain a crude product of 2-chloro-5-trifluoromethylpyridine; heating to melt the crude product of 2-chloro-5-trifluoromethylpyridine; refrigerating and circulating in a low-temperature thermostat; cooling and crystallizing and separating to obtain recrystal; sweating the recrystal; repeating the processes of circulating, melting, recrystallizing and sweating to obtain high-purity 2-chloro-5-trifluoromethylpyridine. According to the production method provided by theinvention, 3-methylpyridine is adopted as a raw material, and the 2-chloro-5-trifluoromethylpyridine is molten, recrystallized and purified so that the product has high purity and stable yield.
Owner:SUZHOU GAIDE FINE MATERIALS CO LTD
Preparation of 3-cyanopyridine
A process for preparing 3-cyanopyridine from 3-methylpyridine, ammonia gas and O2 features its catalyst has the formula: V1.0CraAbBcCdOx, where A is chosen from P, B, Bi, Sb and As, B is chosen from alkali metal and alkali-earth metal, and C is chosen from Mn, Ti, Ni, Co, Pb, Fe, Mo, W and RE. Its advantage is high output rate of target product.
Owner:CHINA PETROLEUM & CHEM CORP +1
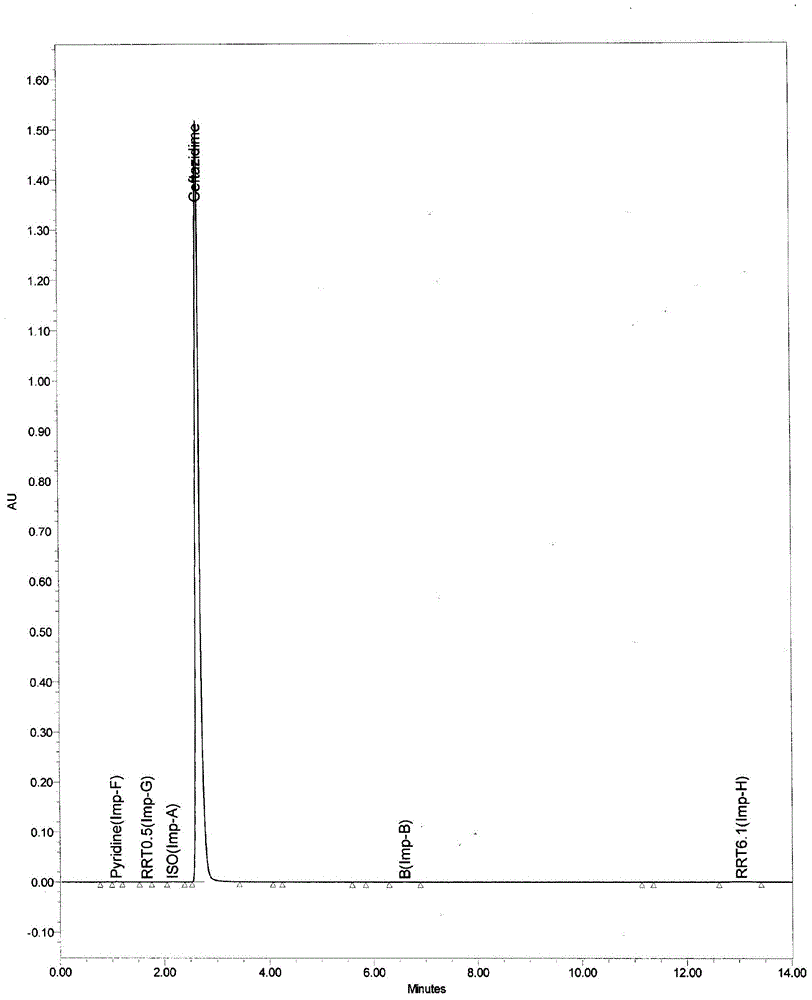
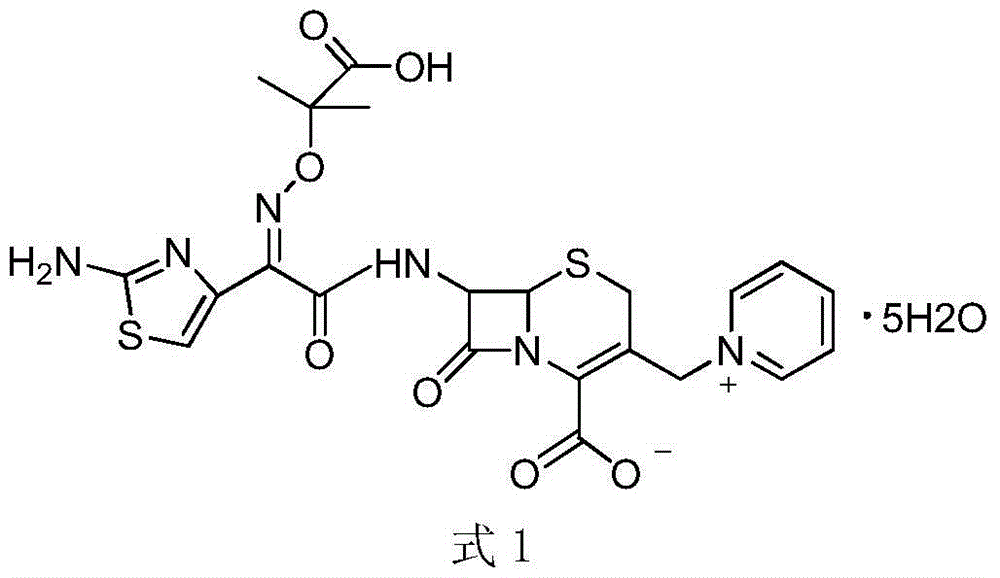
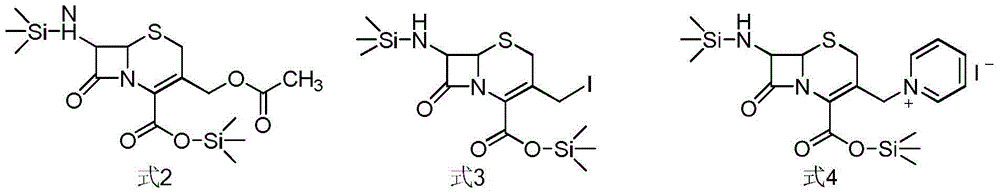
Method for preparing ceftazidime by one-pot process
The invention relates to a method for preparing ceftazidime by a one-pot process. The method comprises the following steps: by using 7-aminocephalosporanic acid as the raw material, carrying out silanization reaction and iodination reaction, reacting with pyridine, directly adding the liquid into ceftazidime side chain acyl chloride hydrochloride to perform acylation reaction without separation to obtain ceftazidime iodate, adding the liquid into a concentrated hydrochloric acid-water mixed solution to perform deprotection, extracting to stratify, and regulating the pH value of the water phase with an alkaline solution to obtain ceftazidime (6R,7R)-7-[[(2-amino-4-thiazolyl)-[(1-carboxy-1-methylethoxy)imino]acetyl]amino]-2-carboxy-8-oxo-5-thia-1-azabicyclo[4,2,0]octyl-2-ene-3-methylpyridine pentahydrate. The method has the advantages of high yield, low cost, mild technological conditions, controllable technical process, high safety and low energy consumption, and is simple to operate.
Owner:QILU ANTIBIOTICS PHARMA
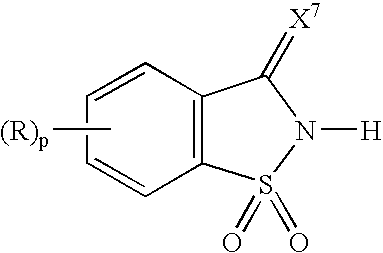
Activators for oligonucleotide synthesis
InactiveUS7501505B2Easy to condenseEasy to useSugar derivativesGroup 5/15 element organic compoundsArylBenzene
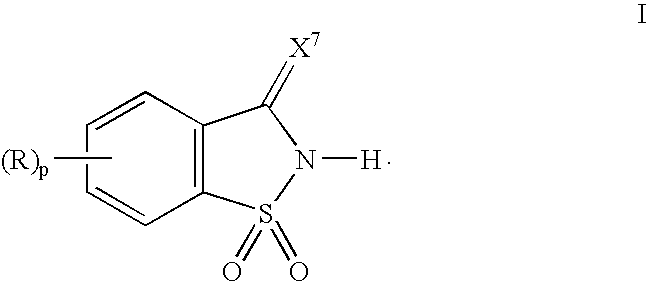
A process for the synthesis of oligonucleotides using phosphoramidite chemistry is provided. The process employs as activator a 1,1-dioxo-1,2-dihydro-1λ6-benzo[d]isothiazol-3-one, preferably in the presence of an organic base. The 1,1-dioxo-1,2-dihydro-1λ6-benzo[d]isothiazol-3-one is represented by the following structural formula:wherein p is 0 or an integer from 1 to 4; X7 is O or S; R for each occurrence is a substituent, preferably each independently, a halo, a substituted or unsubstituted aliphatic group, —NR11R12, —OR13, —OC(O)R13, —C(O)OR13, or cyano; or two adjacent R groups taken together with the carbon atoms to which they are attached form a six membered saturated or unsaturated ring; R11 and R12 are each, independently, —H, a substituted or unsubstituted aliphatic group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aralkyl group; and R13 is a substituted or unsubstituted aliphatic group, a substituted or unsubstituted aryl group, or a substituted or unsubstituted aralkyl group. Preferred organic bases are pyridine, 3-methylpyridine, or N-methylimidazole.
Owner:NITTO DENKO AVECIA
Method of rapidly and quantitatively analyzing 48 amino acids
ActiveCN110018266AImprove extraction efficiencyHigh derivatization efficiencyComponent separationPretreatment methodDerivatization
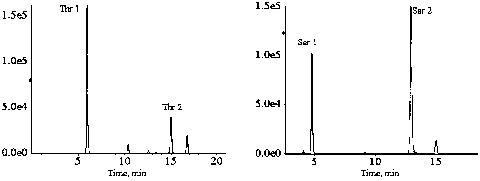
The invention discloses a method of rapidly and quantitatively analyzing 48 amino acids. A derivatization reagent combination for quantitatively analyzing and detecting an amino acid content by a derivatization HPLC-MS / MS method is provided, and comprises a derivatization reagent A and a derivatization reagent B; the derivatization reagent A is a mixed solution of n-propanol and 3-methylpyridine;and the derivatization reagent B is a mixed solution of dichloromethane, chloroformyl propyl ester and isooctane. Furthermore, a stable isotope internal standard solution combination, a sample pretreatment method and a liquid chromatography-tandem mass spectrometry method based on the derivatization method are established. The derivatization reagent proposed by the invention has high derivatization efficiency and low toxicity; the method requires fewer samples, the processing is simple and quick, the repeatability is good, and the detection cost is low; absolute quantitative analysis on 48 amino acids is realized by only 22 minutes, the analysis time is greatly shortened, and the detection throughput is improved; and great promotion and application value are achieved.
Owner:GUANGZHOU WOMEN AND CHILDRENS MEDICAL CENTER
Double-ligand zinc complex catalyst and application thereof
InactiveCN104492488AReduce dosageIdeal yieldOrganic-compounds/hydrides/coordination-complexes catalystsZinc organic compoundsPhosphoniumQuinoline
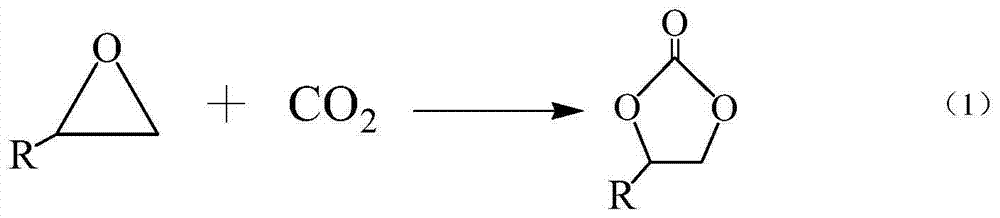
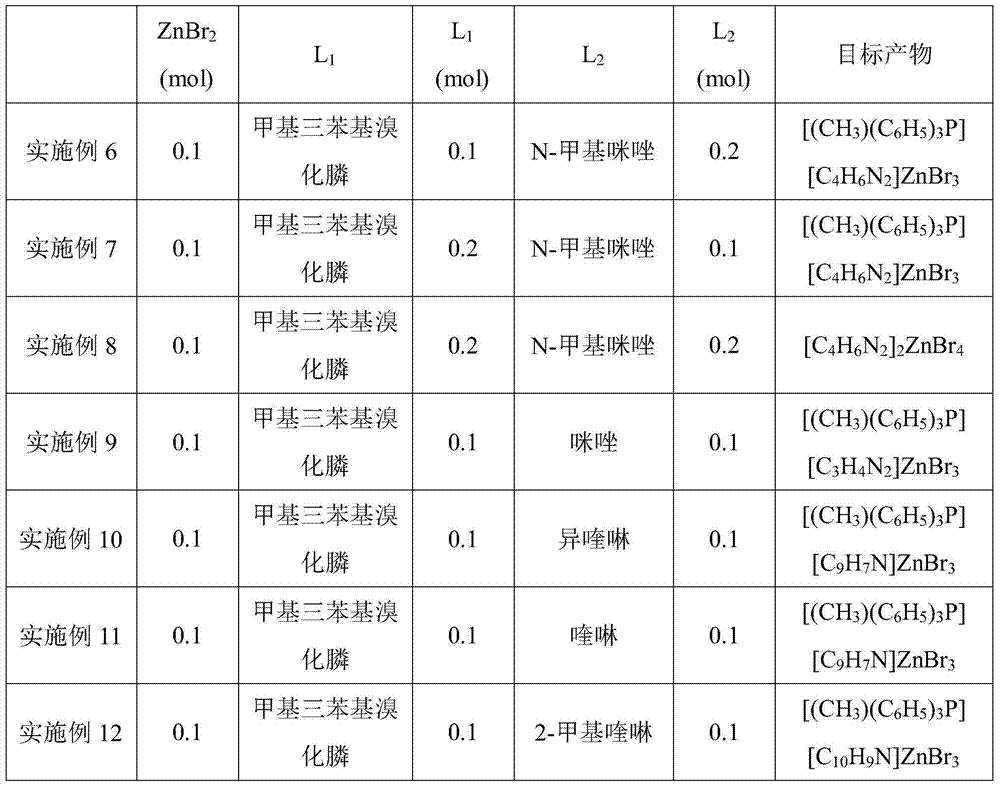
The invention discloses a double-ligand zinc complex catalyst which is prepared from an active main body zinc halide salt, an active functional ligand L1 and a corrosion-inhibition functional ligand L2, wherein the active functional ligand L1 is tetramethyl ammonium halide, tetraethyl ammonium halide, tetrapropyl ammonium halide, tetraethyl phosphonium halide, tetraphenyl phosphonium halide, methyl tri-tert-butyl phosphonium halide or methyl triphenyl phosphonium halide; and the corrosion-inhibition functional ligand L2 is N-methylimidazole, iminazole, isoquinoline, quinoline, 2-toluquinoline, 4-toluquinoline, biquinoline, pyridine, 3-methylpyridine or 1,10-o-phenanthroline. The invention also discloses application of the catalyst in preparing corresponding cyclic carbonate by CO2-epoxy compound cycloaddition. The catalyst has the advantages of low temperature, high catalytic activity, low corrosivity, easy separation, high stability, favorable recovery performance and the like, can form a homogeneous catalytic system, and has favorable industrial application prospects.
Owner:NANJING UNIV OF TECH +2
Synthetic method of 2-chloro-5-chloromethyl pyridine
The invention discloses a synthetic method of 2-chloro-5-chloromethyl pyridine. The synthetic method comprising the following steps: adding an organic solvent, an acid buffering agent solution and an initiator into 3-methylpyridine; adjusting the pH value of the solution in a range of 4-5; letting nitrogen in; elevating temperature to 80-100 DEG C while stirring; stopping letting nitrogen in, letting chlorine in and continuing elevating temperature for a reaction; stopping heating, shutting a chlorine injection vavle off, letting nitrogen in and bubbling to drive the chlorine off; desolventizing a reaction solution through underpressure distillation to obtain brown-red oily liquid; and purifying the brown-red oily liquid to obtain a finished product. According to the invention, a liquid phase chlorination method is adopted; raw materials are subjected to a reaction in liquid; meanwhile an acid buffering agent solution is added to adjust a pH value in a range of 4-5; and by-products are decreased, so that the yield of a target product can reach to about 90%.
Owner:JIANGSU KESHENG CROP TECH
A kind of method of producing pyridine compound
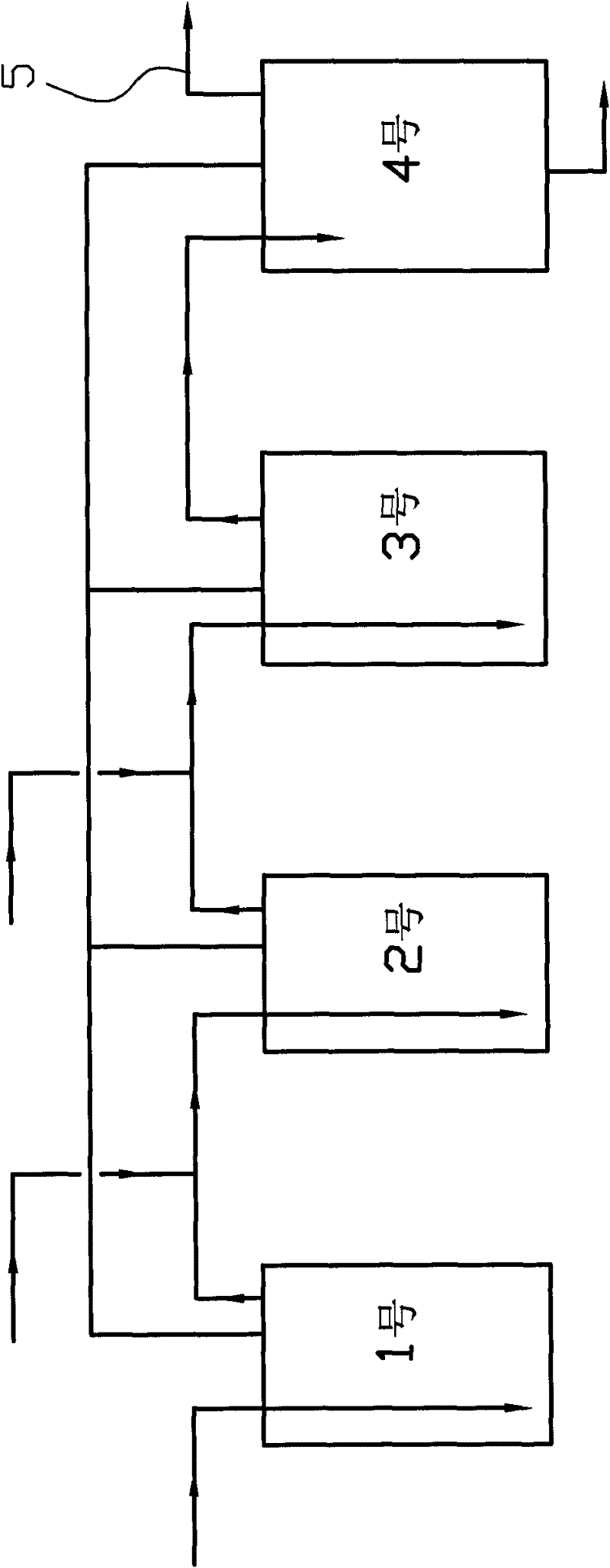
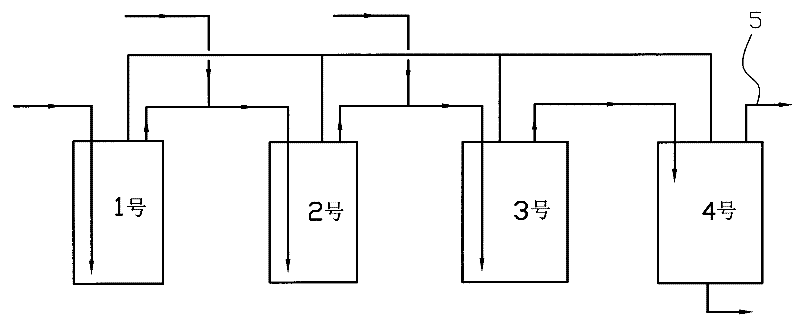
ActiveCN102295595AIncrease profitIncrease productivityOrganic chemistryAqueous solution3-Methylpyridine
The invention relates to a method for producing a pyridine compound. The method comprises the following steps: respectively placing a 50% ethanol aqueous solution in four reaction kettles, respectively placing a solid catalyst in the first three reaction kettles, and simultaneously heating the four reaction kettles to 220-230 DEG C; respectively putting a raw material prepared in advance to the bottoms of the inner cavities of the first three reaction kettles through pipelines; mixing liquid flowing out of the outlet of the NO.1 reaction kettle with the raw material in the NO.2 reaction kettle and then flowing to the bottom of the inner cavity of the NO.2 reaction kettle together; mixing the liquid flowing out of the outlet of the NO.2 reaction kettle with the raw material in the NO.3 reaction kettle and then flowing to the bottom of the inner cavity of the NO.3 reaction kettle together; and enabling the liquid flowing out of the NO. 3 reaction kettle to flow into the NO.4 reaction kettle through the pipeline; controlling the pressure and liquid level of the NO.4 reaction kettle, and continuously feeding material for 300 hours; and distilling the liquid flowing out of the NO.4 reaction kettle so as to obtain the pyridine compound which mainly contains 3-methylpyridine. The method has the advantages that through continuous liquid phase reaction, production efficiency is greatlyimproved, the utilization of equipment is improved, and the utilization of energy is improved to a large degree.
Owner:浙江爱迪亚营养科技开发有限公司
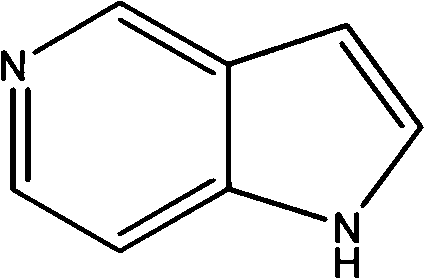
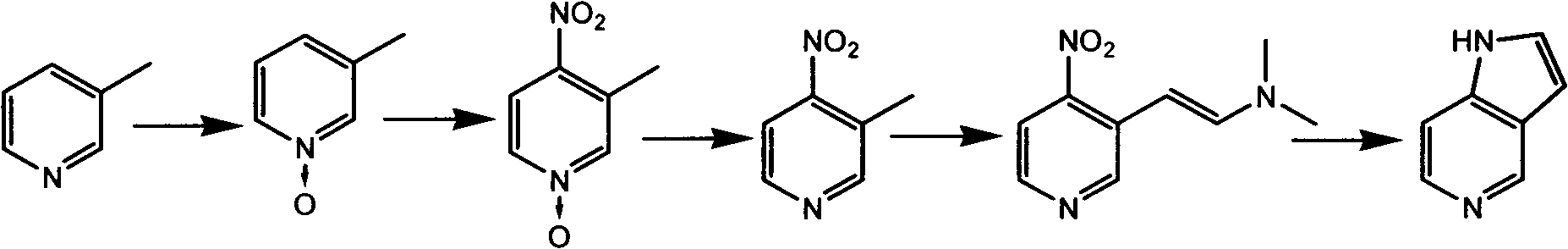
5-azaindole preparation method
InactiveCN103421004ARaw materials are cheap and easy to getReduce manufacturing costOrganic chemistryNitrationMethyl group
The present invention discloses a 5-azaindole preparation method, wherein 3-methylpyridine is adopted as a raw material, and the 5-azaindole preparation method comprises the following five steps that: (1) 3-methylpyridine is subjected to an oxidation reaction under an oxidant effect to obtain a 3-methylpyridine nitrogen oxide, (2) the 3-methylpyridine nitrogen oxide is subjected to a nitration reaction under a mixed acid effect to obtain a 3-methyl-4-nitropyridine nitrogen oxide, (3) the 3-methyl-4-nitropyridine nitrogen oxide and phosphorus trichloride are subjected to a reaction in a solvent to obtain 3-methyl-4-nitropyridine, (4) the 3-methyl-4-nitropyridine and N,N-dimethyl formamide dialkyl acetal are subjected to a reaction in a solvent N,N-dimethyl formamide under a heating condition to obtain 3-dialkylamine vinyl-4-nitropyridine, and (5) the 3-dialkylamine vinyl-4-nitropyridine is subjected to a reduction reaction under a metal catalyst effect while ring closure is performed to obtain the final product 5-azaindole.
Owner:ASCEPION PHARMA
Preparation method of new crystal form of dexrabeprazole sodium
InactiveCN104910135ANo changeGood crystal stabilityOrganic chemistry methodsAlkaline waterFiltration
Owner:NANJING KEFEI PINGSHENGHUI PHARMA CO LTD +2
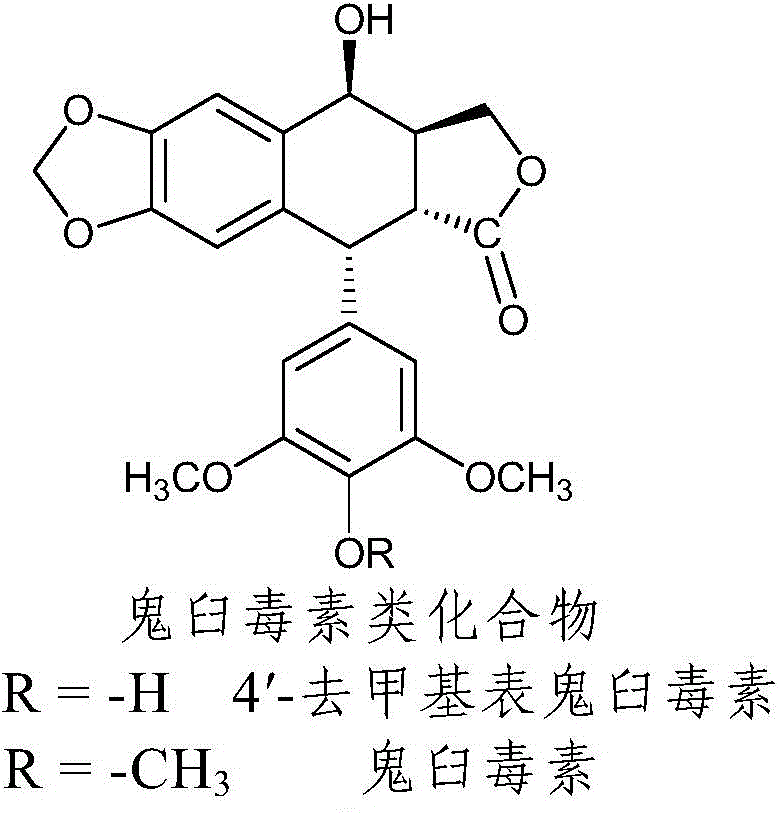
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
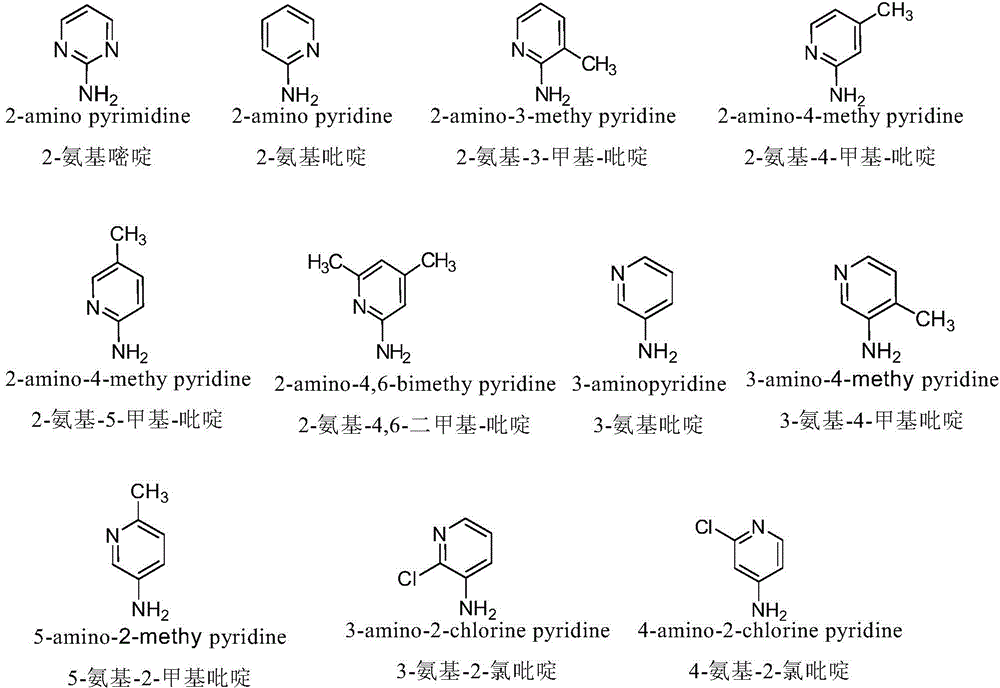
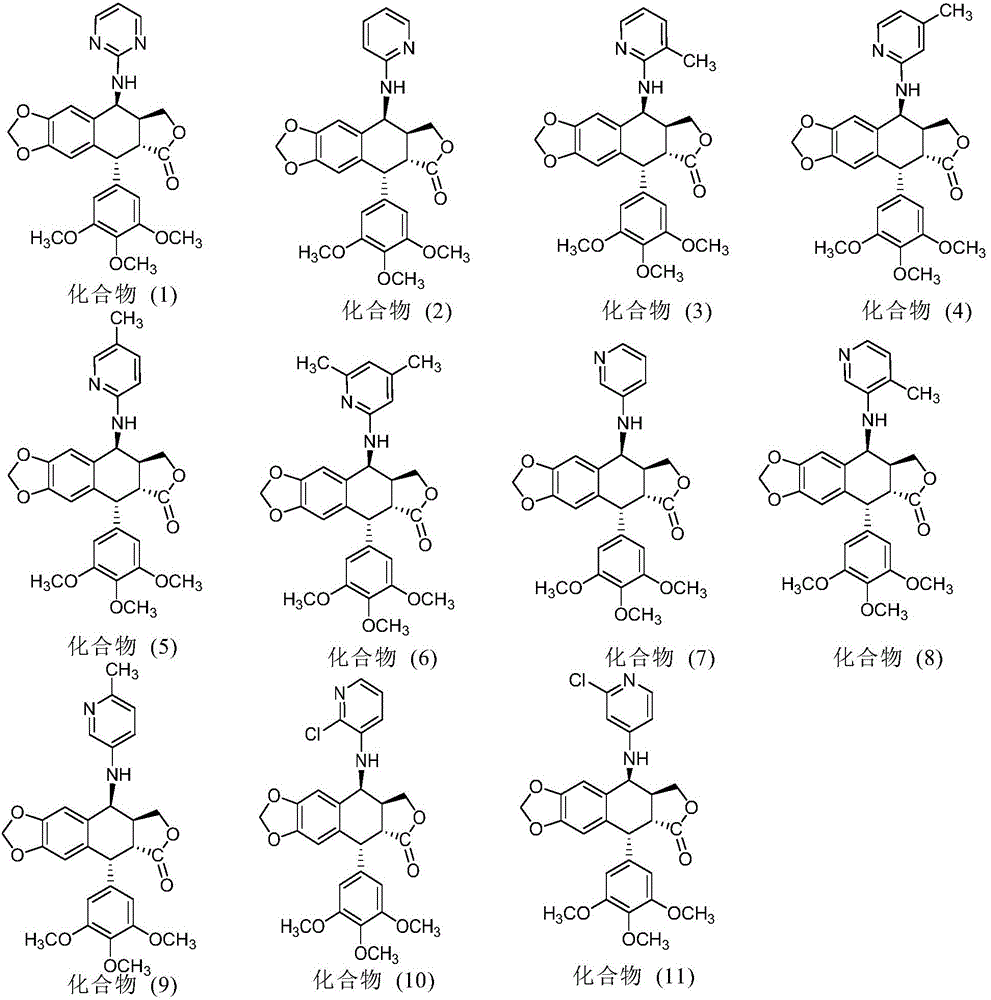
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
Method for synthesizing 2-chloro-5-trifluoromethylpyridine
InactiveCN102452976AImprove operational efficiencyEasy to operateOrganic chemistryBenzoyl chlorideOxygen
The invention relates to a method for synthesizing 2-chloro-5-trifluoromethylpyridine. In the method, the 2-chloro-5-trifluoromethylpyridine is synthesized by performing three-step reaction, namely N-oxidation, benzoyl chloride chlorination and chorine gas chlorination on 3-methylpyridine serving as an initial raw material. In the reaction process of preparing 2-chloro-5-methylpyridine from N-oxy-3-methylpyridine, an isomer 2-chloro-3-methylpyridine of which the property is similar to that of the target product is not required to be separated, and the 2-chloro-5-methylpyridine can be directly used for the next-step reaction, so that the separation process is simplified, and operation efficiency is improved; and the whole synthesis route is easy to operate, reaction conditions are mild, and the requirement on an equipment material is low.
Owner:NINGBO UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b124604c-472f-4724-bf64-0f015ca99da0/US20090170905A1-20090702-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b124604c-472f-4724-bf64-0f015ca99da0/US20090170905A1-20090702-D00002.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/b124604c-472f-4724-bf64-0f015ca99da0/US20090170905A1-20090702-D00003.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e8a14e3a-9ca0-49d2-90b3-1e76d38e43cb/US20130085158A1-20130404-D00000.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e8a14e3a-9ca0-49d2-90b3-1e76d38e43cb/US20130085158A1-20130404-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/e8a14e3a-9ca0-49d2-90b3-1e76d38e43cb/US20130085158A1-20130404-D00002.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/20df7b3e-93e1-42fb-b917-fc26f621d718/US20110263654A1-20111027-D00000.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/20df7b3e-93e1-42fb-b917-fc26f621d718/US20110263654A1-20111027-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/20df7b3e-93e1-42fb-b917-fc26f621d718/US20110263654A1-20111027-D00002.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead979ad-ae5d-4461-be12-b4fd799554d8/US08716338-20140506-D00001.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead979ad-ae5d-4461-be12-b4fd799554d8/US08716338-20140506-D00002.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[D] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/ead979ad-ae5d-4461-be12-b4fd799554d8/US08716338-20140506-D00003.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00001.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00002.png)
![Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Solid forms of 3-(6-(1-(2,2-difluorobenzo[d][1,3]dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/2227a22a-6599-41cb-afc0-116ff5abe172/US20120277268A1-20121101-D00003.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78eab925-31b8-4f59-aa4c-ebe2cbc8024d/US20140206720A1-20140724-D00001.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78eab925-31b8-4f59-aa4c-ebe2cbc8024d/US20140206720A1-20140724-D00002.png)
![Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid Dosage units of 3-(6-(1-(2,2-difluorobenzo[d] [1,3] dioxol-5-yl) cyclopropanecarboxamido)-3-methylpyridin-2-yl)benzoic acid](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/78eab925-31b8-4f59-aa4c-ebe2cbc8024d/US20140206720A1-20140724-D00003.png)
![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00001.png)
![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00002.png)
![Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides Process for the preparation of N-([1,2,4]triazolopyrimidin-2-yl)aryl sulfonamides](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/c96bac2d-a089-48c3-9093-72ced3214ea5/US20050215570A1-20050929-C00003.png)
![Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cdb8d835-4e80-4d0c-89ee-5661e1f7c489/FDA0000058962890000011.png)
![Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cdb8d835-4e80-4d0c-89ee-5661e1f7c489/FDA0000058962890000012.png)
![Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors Pyrazolo[3,4-d]pyrimidinone compounds and their application in the preparation of phosphodiesterase ⅸ inhibitors](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/cdb8d835-4e80-4d0c-89ee-5661e1f7c489/FDA0000058962890000021.png)