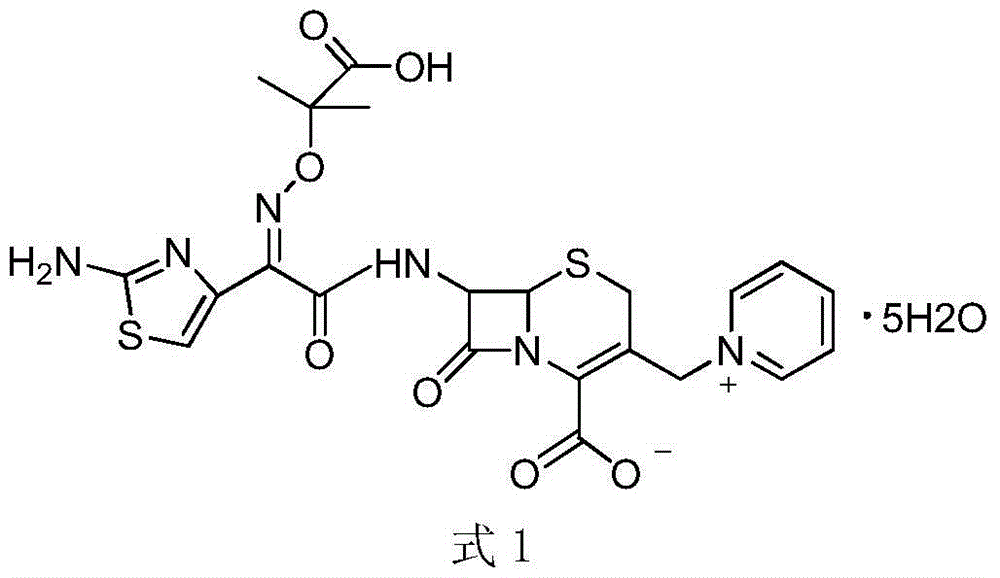
Method for preparing ceftazidime by one-pot process
A technology of ceftazidime and aminocephalosporanic acid, which is applied in the field of drug synthesis, can solve problems such as product loss, waste of excipients, and impact on the overall yield of ceftazidime, and achieve the effects of increased product yield, high safety, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1, the preparation of ceftazidime:
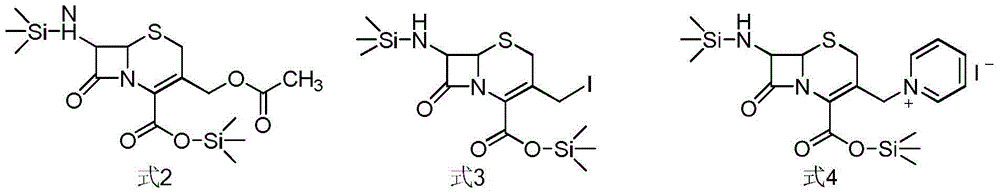
[0049] 30 g (0.11 mol) of 7-aminocephalosporanic acid, 300 mL of dichloromethane, and 35 mL (0.16 mol) of hexamethyldisilazane were placed in a reaction flask, heated to reflux for 8 hours, and N,N-diethyl ether was added under ice bath 29mL (0.18mol) of base aniline, 32g (0.16mol) of trimethylsilyl iodide, react at room temperature for 3hr, add pyridine 18mL (0.22mol) under ice bath, continue to react for 1hr;
[0050] The above feed liquid was cooled to -25°C, 39 g (0.12 mol) of ceftazidime side chain acid chloride hydrochloride (compound of formula 5) was added in batches, and the reaction was incubated for 2 h after the addition;
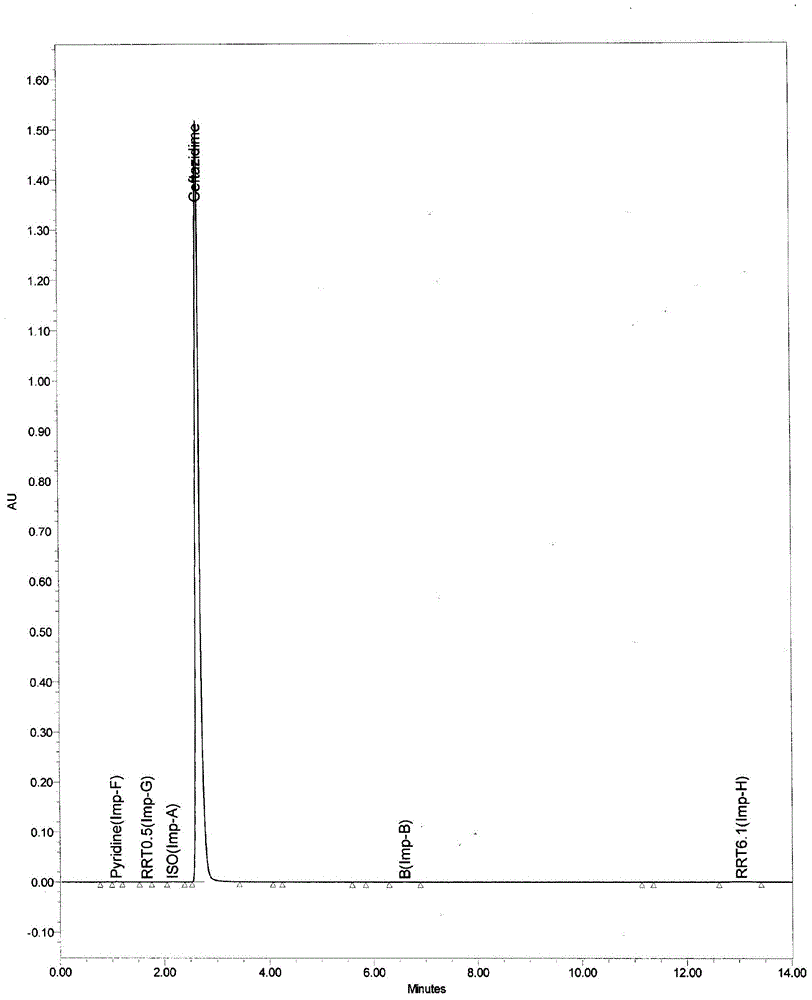
[0051] The temperature is controlled at 0±5°C, the above-mentioned reaction feed liquid is slowly transferred into the mixed solution of 50mL concentrated hydrochloric acid / 50mL water (the solution is pre-cooled to 0°C in advance), the temperature is kept and stirred until it is completely d...
Embodiment 2
[0052] Embodiment 2, the synthesis of ceftazidime:
[0053] 40 g of 7-aminocephalosporanic acid, 300 mL of dichloromethane, and 95.5 mL (0.39 mol) of N,O-bistrimethylsilylacetamide were placed in a reaction flask, heated to reflux for 8 hours, and N,N-dimethylacetal was added under ice bath. 40 mL (0.24 mol) of phenylaniline, 43 g of trimethylsilyl iodide, react at room temperature for 3 h, add 24 ml (0.29 mol) of pyridine under ice bath, and continue to react for 1 h;
[0054] The above-mentioned feed liquid was cooled to -25°C, 72 g (0.28 mol) of ceftazidime side chain acid chloride hydrochloride (compound of formula 5) was added in batches, and the reaction was incubated for 2 h after the addition;
[0055] The temperature is controlled at 0±5°C, the above-mentioned reaction feed liquid is slowly transferred into the mixed solution of 70mL concentrated hydrochloric acid / 70mL water (the solution is pre-cooled to 0°C in advance), the temperature is kept and stirred until it i...
Embodiment 3
[0056] Embodiment 3, the synthesis of ceftazidime:
[0057] As described in Example 1, the difference is that the silanizing agent is 39.2 mL (0.31 mol) of trimethylchlorosilane, the acid scavenging agent is 12.3 mL (0.09 mol) of triethylamine, and the pH value of the aqueous phase after extraction is adjusted with 10 % (mass percent) sodium hydroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com