Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
A technology of anti-tumor activity and pyridine derivatives, applied in the preparation and application of podophyllotoxin derivatives, nitrogen-substituted podophyllin derivatives and their preparation, nitrogen-substituted podophyllin derivatives in the preparation of anti-tumor In the field of medicine, it can solve problems such as limited application, high toxicity and side effects, and poor bioavailability, and achieve the effect of improving anti-tumor activity and good anti-tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
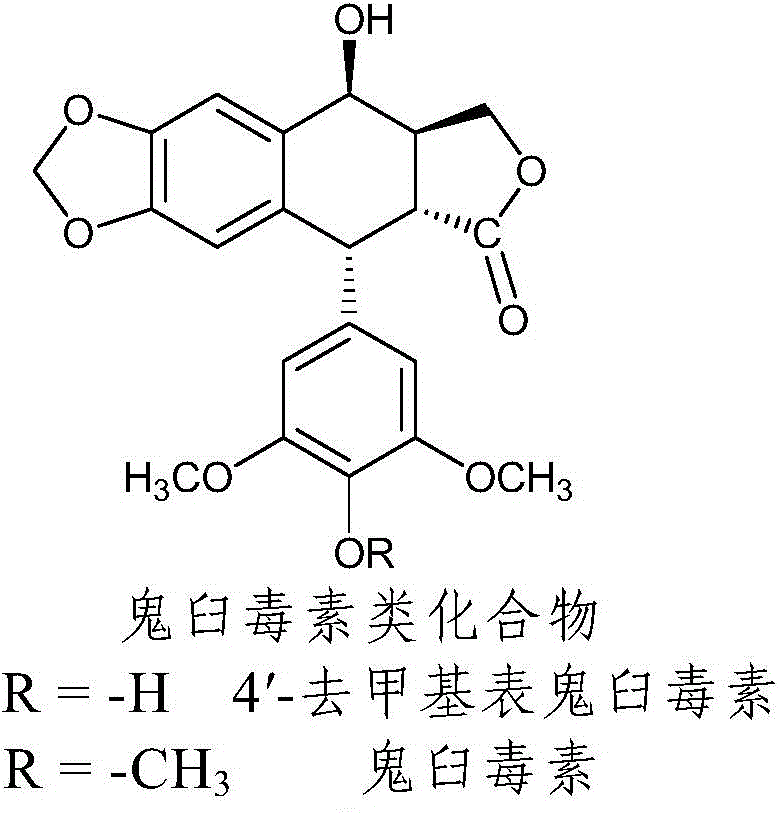
[0039] Preliminary Example 1 Activation of Podophyllotoxin C-ring 4
[0040] Drying of dichloromethane: Weigh 1.5g of calcium hydride into a 1000ML round-bottom four-necked bottle, put a clean funnel into the side port, pour 500ML of dichloromethane, add 3-4 glass beads to prevent bumping, and adjust the heating When the temperature reaches the dichloromethane in a slightly boiling state, add a reflux tube to reflux for 2-3 hours, then condense and recover it into a reagent bottle filled with anhydrous calcium chloride. After collecting the liquid, put a little nitrogen into the bottle, and close the lid. Yes, you can add nitrogen after each use.
[0041] Weigh 2 grams of podophyllotoxin or 4′-desmethyl epipodophyllotoxin, and vacuum-dry at 45°C for 2 hours; under nitrogen protection, add the dried podophyllotoxin and 40ml of dried dichloromethane into a 250ml four-necked bottle , add 10ml of hydrogen bromide and react in ice bath for half an hour. After the reaction, extrac...
Embodiment 2
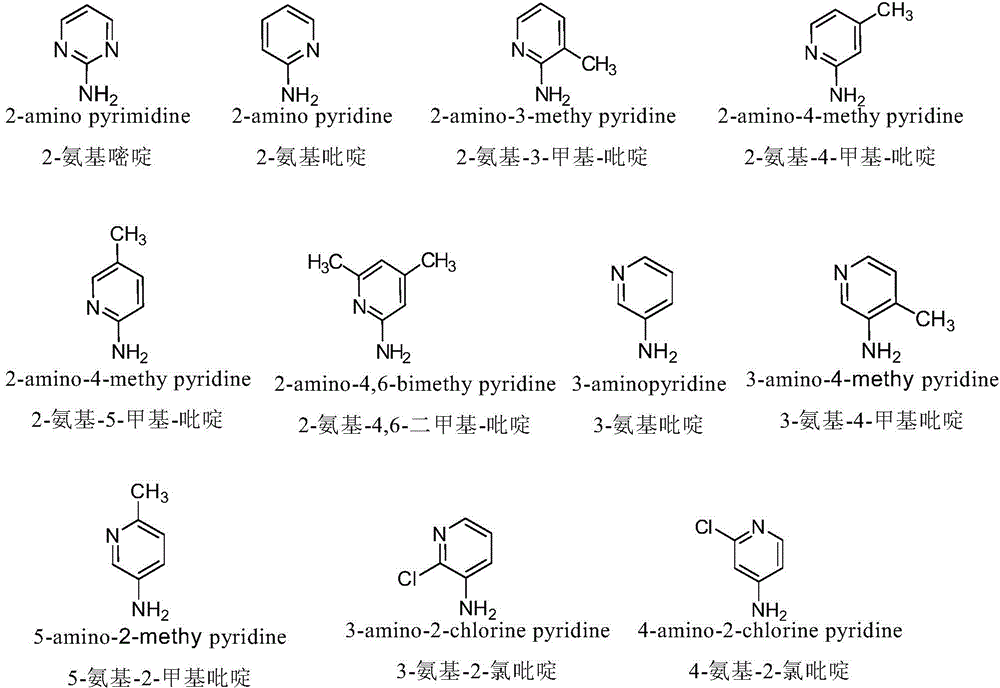
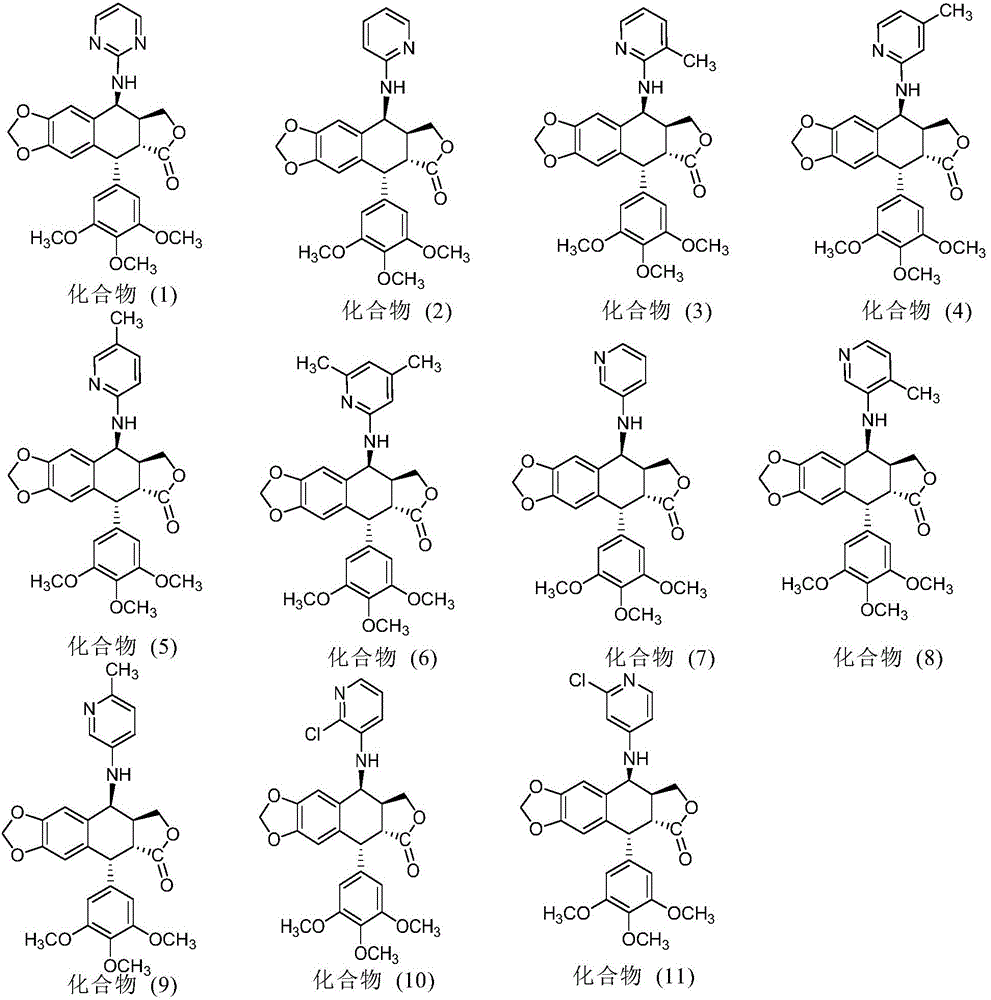
[0051] Example 2 Synthesis and purification of 4-N-(2-aminopyridine)-4-deoxy-podophyllotoxin (compound (2))
[0052] (1) Synthesis of 4-N-(2-aminopyridine)-4-deoxy-podophyllotoxin: Weigh 1 mol of the activated product at position 4 of the C-ring of podophyllotoxin (prepared in Preliminary Example 1), and vacuum-dry at 45°C 2 hours; under the protection of nitrogen, add the dried dichloromethane into the four-necked flask, add the activated product of the 4-position of the C ring of the dried podophyllotoxin compound and 1.2mol of 2-aminopyridine, and then add 2mol of barium carbonate, at room temperature Under the conditions, the reaction was stirred for 48 hours, and the reaction solution was spin-dried to obtain a crude product of 4-N-(2-aminopyridine)-4-deoxy-podophyllotoxin.
[0053] (2) Separation and purification of 4-N-(2-aminopyridine)-4-deoxy-podophyllotoxin:
[0054] Separation and purification using silica gel column chromatography and gel column chromatography:
...
Embodiment 3
[0060] Example 3 Synthesis and purification of 4-N-(2-amino-3-picoline)-4-deoxy-podophyllotoxin (compound (3))
[0061](1) Synthesis of 4-N-(2-amino-3-picoline)-4-deoxy-podophyllotoxin: Weigh 1 mol of the activated product at position 4 of the C-ring of podophyllotoxin (prepared in Preliminary Example 1) , vacuum-dry at 45°C for 2 hours; under nitrogen protection, add the dried dichloromethane into a four-necked flask, add the dried activated product of the 4-position of the C ring of the podophyllotoxin compound and 1.2mol of 2-amino-3-methanol base pyridine, then add 2mol barium carbonate, under normal temperature conditions, stir and react for 48 hours, and the reaction solution is spin-dried to obtain 4-N-(2-amino-3-methylpyridine)-4-deoxy-podophyllotoxin crude product.
[0062] (2) Separation and purification of 4-N-(2-amino-3-picoline)-4-deoxy-podophyllotoxin:
[0063] Separation and purification using silica gel column chromatography and gel column chromatography:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com