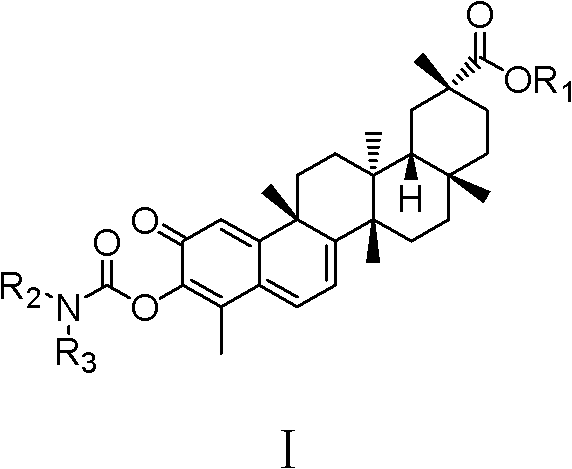
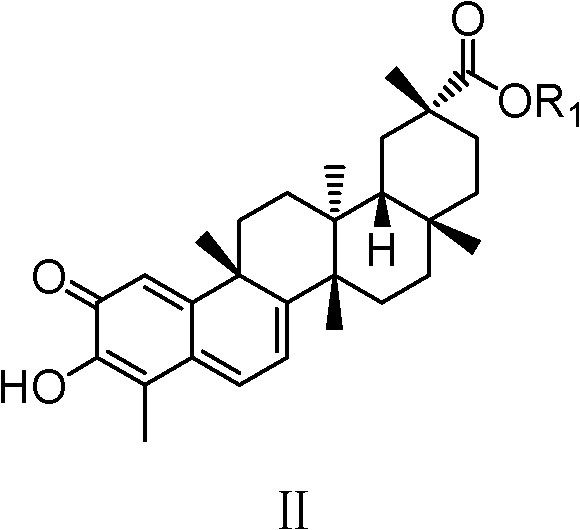
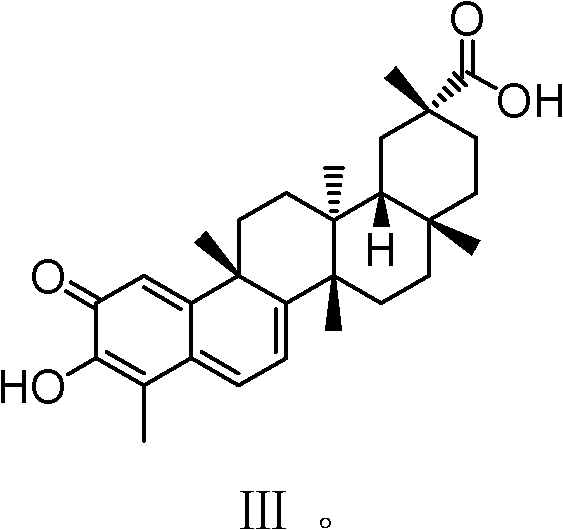
Celastrol derivative and preparation method thereof and application of celastrol derivative to preparation of antitumor medicine
A technology of triptolide and triptolide ester is applied in the application field of triptolide derivatives in the treatment and preparation of antitumor drugs, and can solve the problem of finished medicines without taking relevant consideration and ignoring the water solubility of triptolide problems, etc., to achieve the effect of being conducive to quality control, improving pharmacokinetic properties, and improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Synthesis of tripterine derivative CLS-1 (synthesis route as shown in Figure 1)
[0033] Dissolve 72.8 mg of triphosgene (BTC) (0.24 mmol) in 2 mL of dichloromethane (DCM), add dropwise 1 mL of 0.35 mol / L morpholine (0.35 mmol) dichloromethane solution and 100 μL of triethyl Amine (0.99mmol), stirred at room temperature for 1h, then added dropwise 1mL tripterine (0.2mmol) dichloromethane solution with a concentration of 0.2mol / L, continued to stir for 24h, stopped the reaction, added 15mL deionized water, ethyl acetate Extract 4mL*3 times, combine the organic layers, wash with saturated NaCl solution 4mL*3 times, anhydrous NaCl 2 SO 4 Drying, suction filtration, the filtrate was concentrated to obtain a dark red oily crude product, and then the crude extract was eluted by flash column chromatography with a mixed solvent of n-hexane:acetone volume ratio of 3:1 as the eluent, and spot plate Detect (developing agent petroleum ether: acetone=2: 1; Rf value 0.38...
Embodiment 2
[0038] Synthesis of embodiment 2 tripterine derivatives CLS-2 (synthetic route diagram as shown in Figure 1)
[0039] Dissolve 72.8 mg of BTC (0.24 mmol) in 2 mL of dichloromethane (DCM), add 1 mL of 0.35 mol / L N-methylpiperazine (0.35 mmol) dichloromethane solution and 100 μL of triethyl ether dropwise under ice cooling Amine (0.99mmol), stirred at room temperature for 1h, then added 1mL tripterine (0.2mmol) dichloromethane solution with a concentration of 0.2mol / L, continued to stir for 24h, then stopped the reaction, added 15mL deionized water, extracted with ethyl acetate 4mL*3 times, combined organic layer, washed with saturated NaCl solution 4mL*3 times, anhydrous NaCl 2 SO 4 Drying, suction filtration, and concentration to obtain a dark red oily crude product, then the crude extract was eluted by flash column chromatography with a mixed solvent of n-hexane: acetone volume ratio of 3: 1 as eluent, and spot plate detection ( Developing agent sherwood oil: acetone=2: 1; ...
Embodiment 3
[0040] Example 3 Synthesis of tripterine derivatives CLS-3 (synthesis route as shown in Figure 1)
[0041] Dissolve 72.8 mg of triphosgene BTC (0.24 mmol) in 2 mL of dichloromethane (DCM), add dropwise 1 mL of 0.35 mol / L N-ethylpiperazine (0.35 mmol) in dichloromethane solution and 100 μL of Triethylamine, stirred at room temperature for 1h, then added dropwise 1mL of tripterine (0.2mmol) dichloromethane solution with a concentration of 0.2mol / L, continued to stir for 24h, then stopped the reaction, added 15mL of deionized water, and extracted 4mL with ethyl acetate *3 times, combine the organic layers, wash with 4mL of saturated NaCl solution*3 times, anhydrous NaCl 2 SO 4Drying, suction filtration, and concentration to obtain a dark red oily crude product, then the crude extract was eluted by flash column chromatography with a mixed solvent of n-hexane: acetone volume ratio of 3: 1 as eluent, and spot plate detection ( Developing agent sherwood oil: acetone=2: 1; Rf value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com