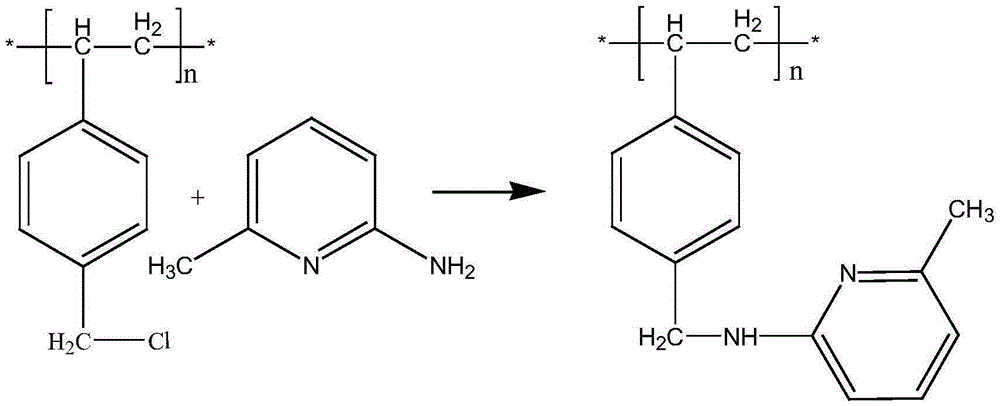
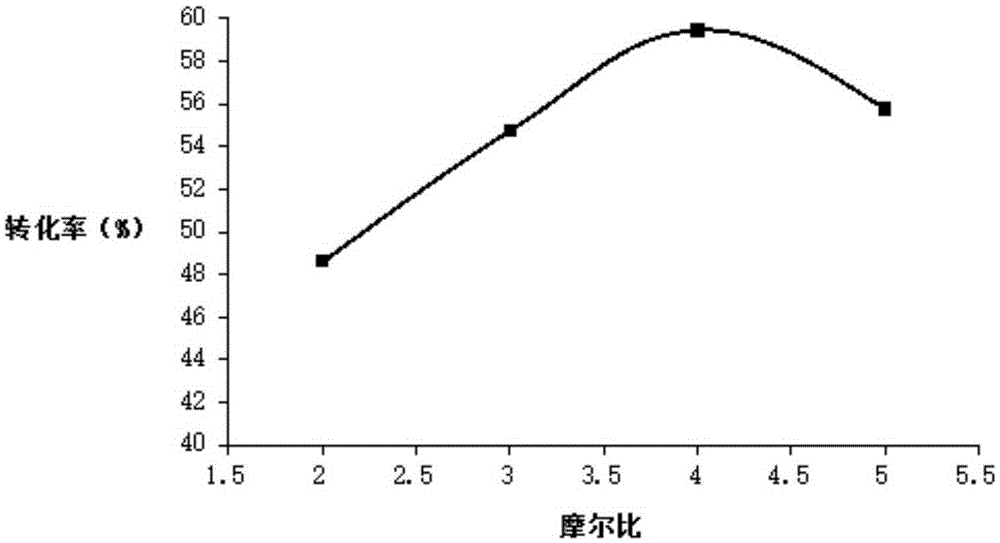
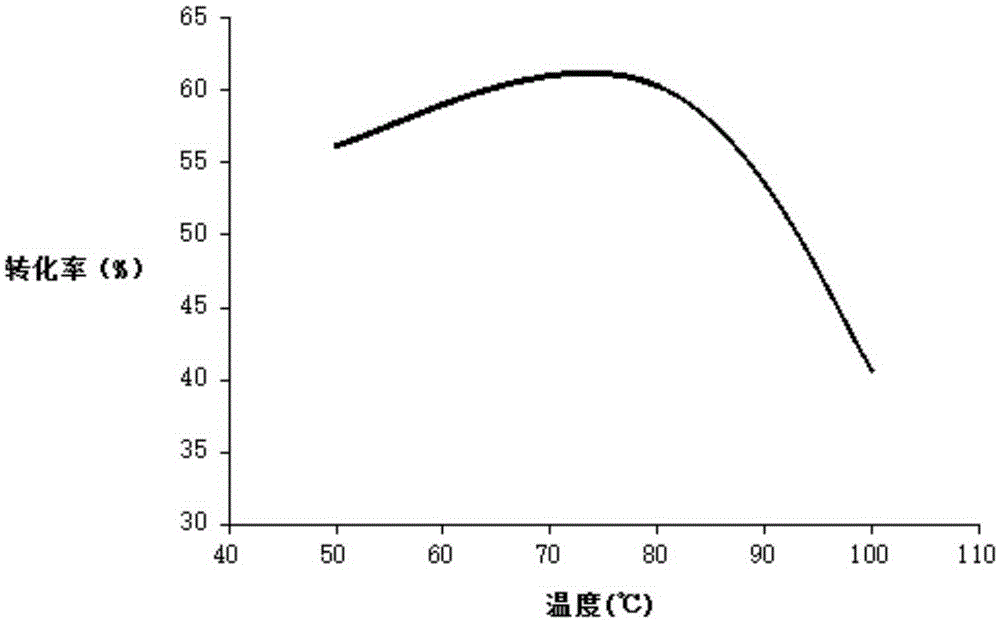
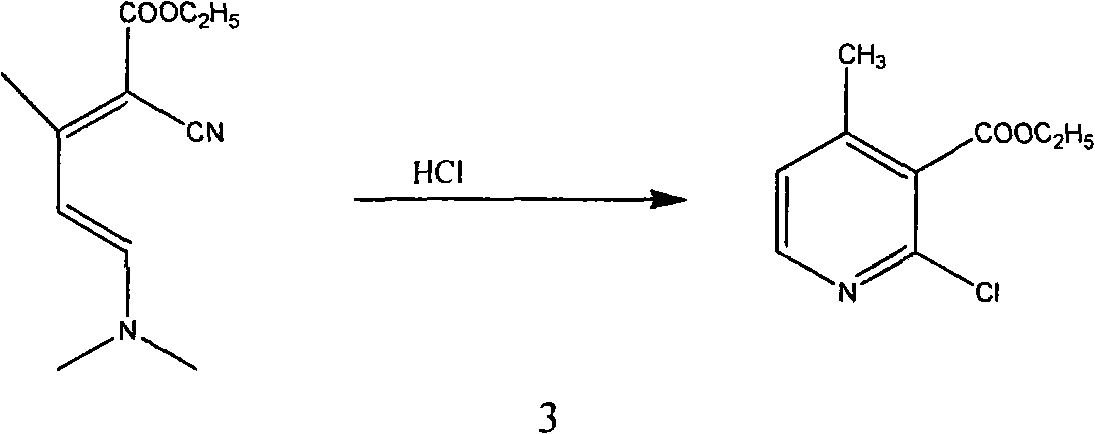
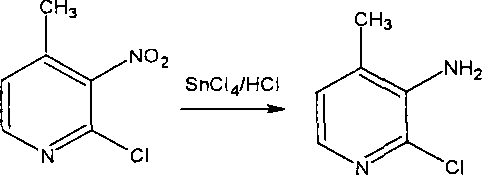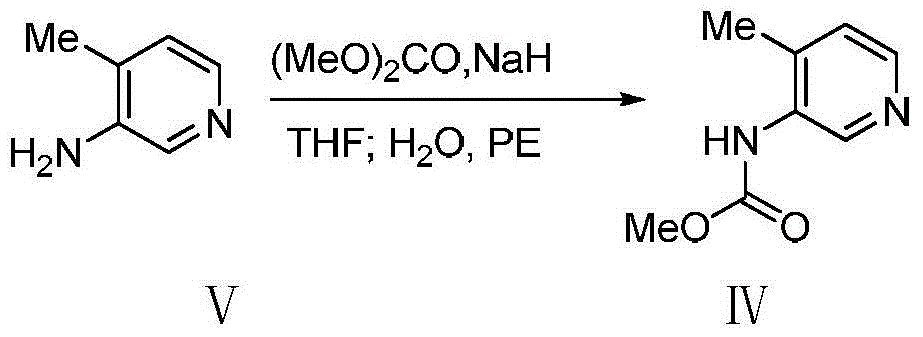Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
131 results about "4-Methylpyridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
4-Methylpyridine is the organic compound with the formula CH₃C₅H₄N. It is one of the three isomers of methylpyridine. This pungent liquid is a building block for the synthesis of other heterocyclic compounds. Its conjugate acid, the 4-methylpyridinium ion, has a pKₐ of 5.98, about 0.7 units above that of pyridine itself.
Antiviral agents and methods of treating viral infections
InactiveUS20020188011A1Enhance the beneficial effectEasy to modifyBiocideCarbohydrate active ingredients4-Methylpyridine3-Aminopyridine
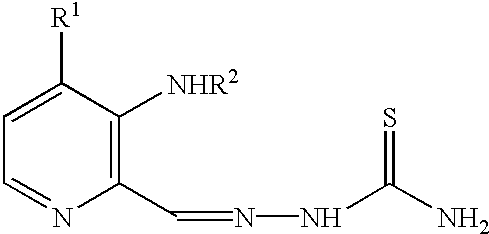
The present invention relates to methods of treating viral or fungal infections using 3-aminopyridine-2-carboxyaldehyde thiosemicarbazone (3-AP) and 3-amino-4-methylpyridine-2-carboxaldehyde thiosemicarbazone (3-AMP) and its prodrug forms and to pharmaceutical compositions comprising these compounds.
Owner:VION PHARMA INC +1
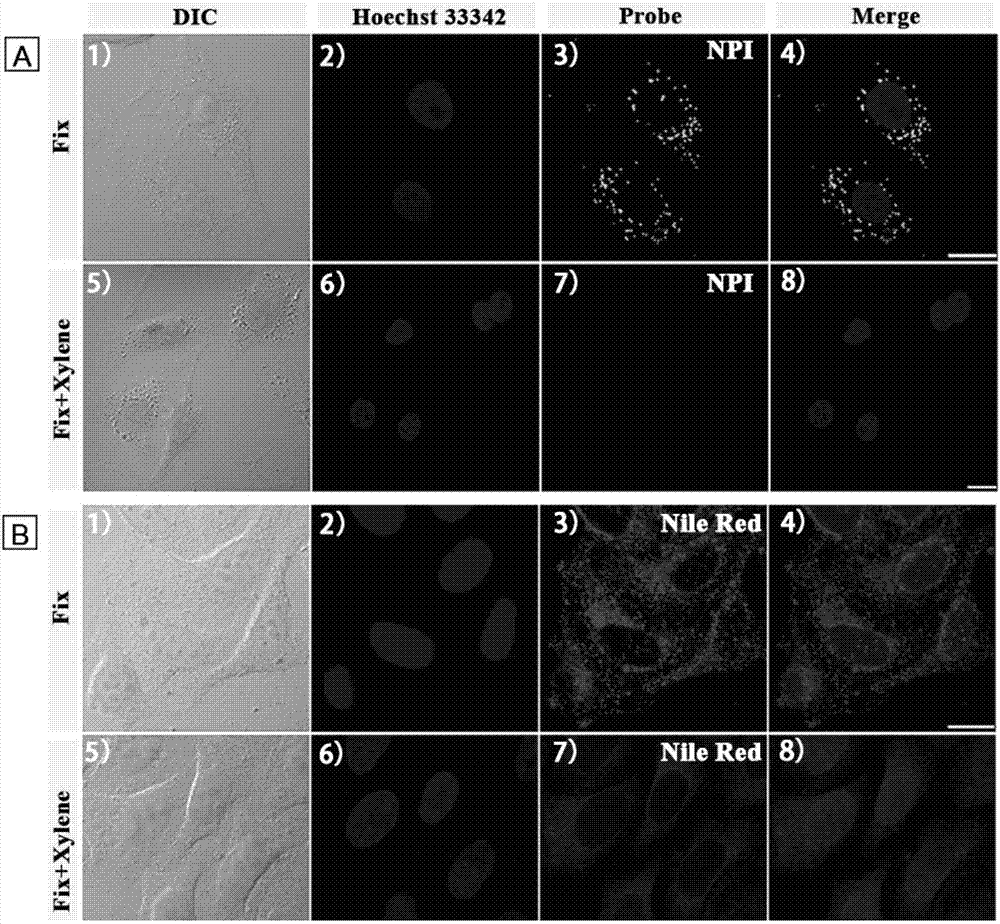
Cell lipid droplet specific-labeling fluorescent probe
InactiveCN106946869AHigh selectivityCapable of two-photon imagingOrganic chemistryFluorescence/phosphorescenceFluorescenceCytotoxicity
The invention discloses a cell lipid droplet specific-labeling fluorescent probe. The fluorescent probe has a chemical name of 1-(3'-(7'-nitrobenzofuran-4'-)aminopropyl)-4-methylpyridine bromide and has a general chemical formula shown in the formula (I). The invention also discloses a use of the fluorescent probe in specific labeling or display of a lipid droplet form and distribution in living cells. An experiment result shows that the cell lipid droplet specific-labeling fluorescent probe has the characteristics of very high selectivity, background noise-free imaging, two-photon performances, very good light stability, fast dyeing ability, no use of washing and low cytotoxicity and has a great application prospect.
Owner:SHANDONG UNIV
Hemicyanine fluorescent dye and preparation method and application thereof
InactiveCN103525117ABright colorImprove visibilityStyryl dyesOrganic chemistry4-MethylpyridineFluorescence
The invention discloses a hemicyanine fluorescent dye and a preparation method and application of the hemicyanine fluorescent dye. The hemicyanine fluorescent dye is obtained through the steps of adding 4-methylpyridine and N, N-diethyl-benzaldehyde into n-butanol according to the mole ratio of one to one, enabling the mixture to react for 4 hours to 6 hours at the temperature ranging from 100 DEG C to 105 DEG C, then, adding 2-chloroethylaminehydrochloride with the molar weight same as the molar weight of the 4-methylpyridine to the solution, enabling the mixture to react at the temperature ranging from 75 DEG C to 80 DEG C, and conducting column chromatography isolation. The hemicyanine fluorescent dye is simple in preparation process and high in productivity, and has good linear optical performance and non-linear optical performance. The dye simultaneously contains cation dye functional groups N<+> and crosslinking dye functional groups NH[2], can be used for dyeing of acrylic fibers, cotton fibers, silk fibers and wool fibers, and is wide in application range. The produced obtained through dyeing is bright in color and good in fluorescence effect.
Owner:SUZHOU UNIV
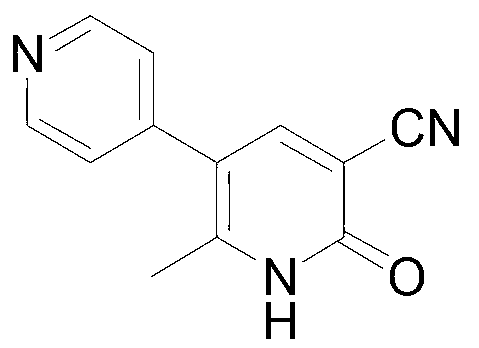
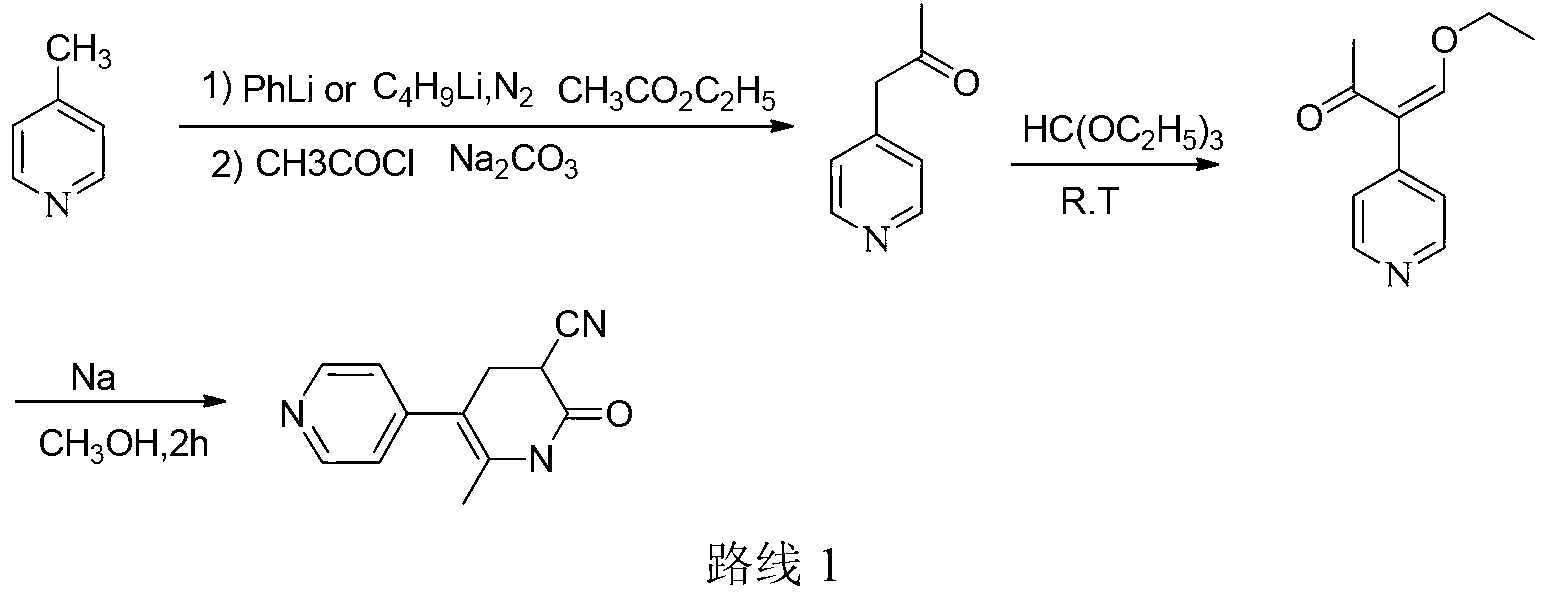
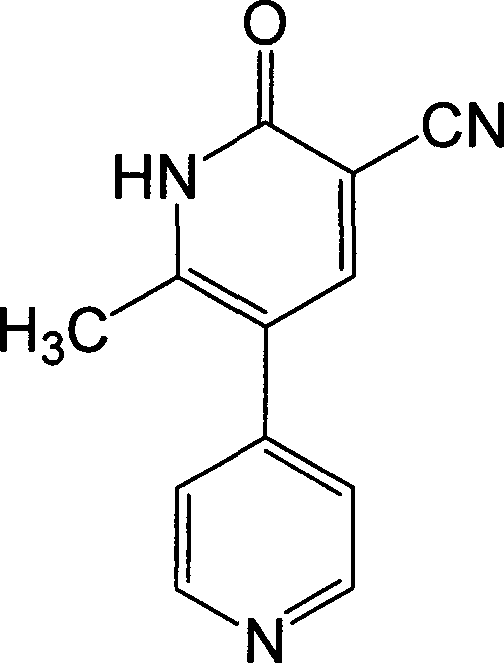
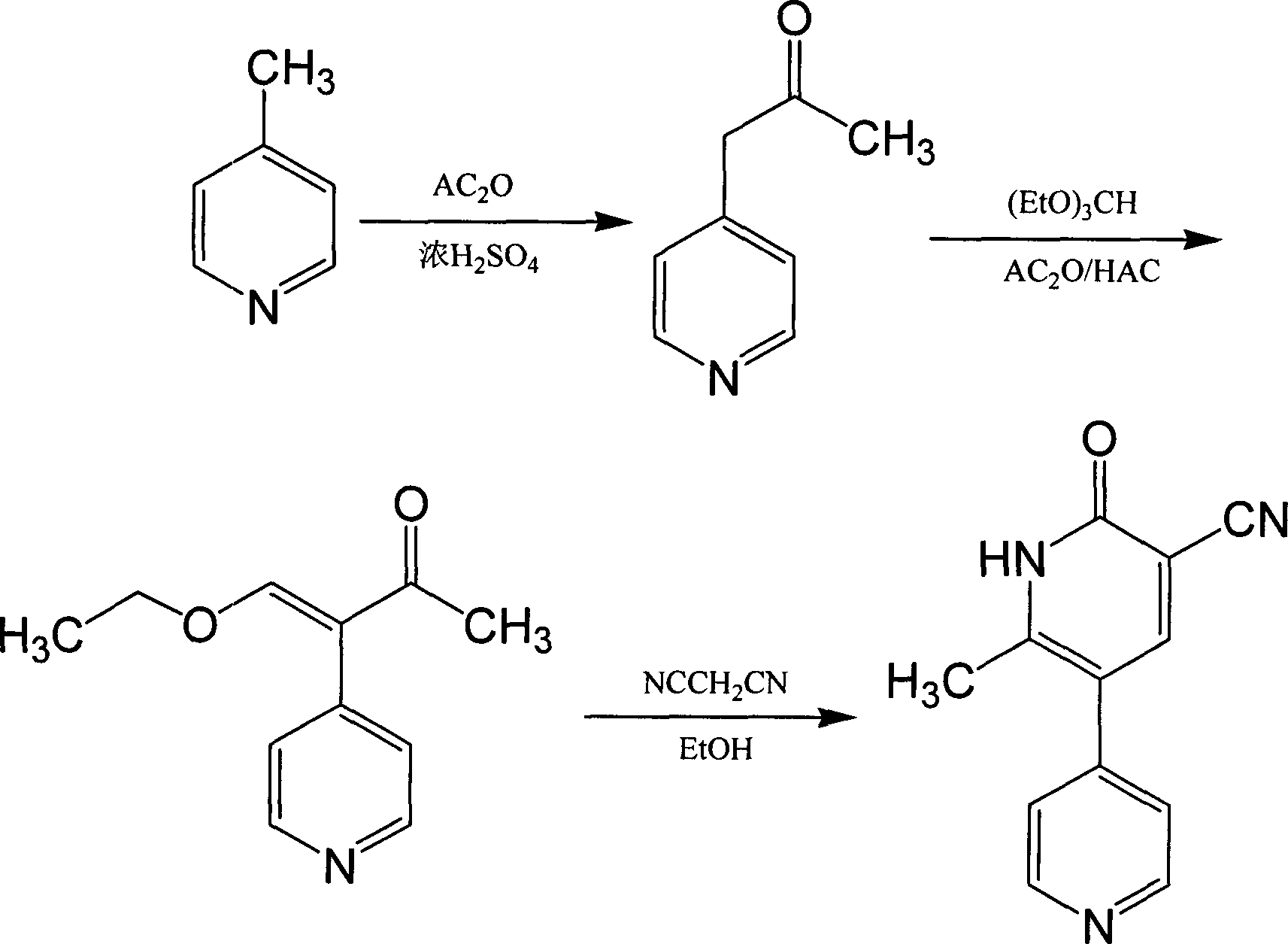
Method for synthesising milrinone
ActiveCN103288725ALow costThe number of times of extraction and decompression concentration is reducedOrganic chemistryAcetic anhydrideSodium bisulfate
The invention discloses a method for synthesising milrinone. The method comprises the following steps of: mixing 4-methylpyridine with acetylchloride in a solvent at a temperature below 10 DEG C; heating to react with or without a catalyst, and then adjusting the pH value of the reaction solution to 7-8 by sodium hydroxide aqueous solution; then directly adding saturated sodium hydrogen sulfite aqueous solution, and reacting; adjusting the pH value of the water layer obtained by the reaction by sodium hydroxide, and reacting to obtain a compound in formula (III); mixing the compound in formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and then reacting at 50-100 DEG C to obtain a compound in formula (IV); cyclizing the compound in formula (IV) with alpha-cyanoacetamide in an alkaline condition to obtain a compound in formula (V). The process is more moderate in reaction conditions, simpler and more convenient to operate, and capable of greatly shortening the original reaction time, reducing cost and increasing yield simultaneously; the process is a preparation method for milrinone which is suitable for industrialized production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
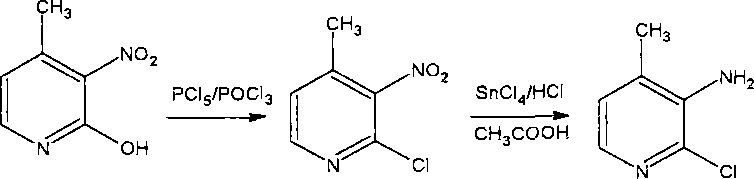
Method for preparing 2-chlorine-3-amino-4-picoline
InactiveCN102898361ARaw materials are easy to getFair priceOrganic chemistry4-MethylpyridineNitration
The invention relates to a novel synthetic method for preparing 2-chlorine-3-amino-4-picoline, and belongs to a production process and corresponding various process conditions for preparing the 2-chlorine-3-amino-4-picoline by using 4-picoline as raw materials sequentially through four-step reaction: concentrated nitric acid nitration, sodium pyrosulfite nitro migration, catalyst catalytic hydrogenation and hydrogen peroxide and concentrated hydrochloric acid chlorination. The method has the beneficial effects that industrial products of 4-picoline are used as raw materials, the raw materials are easily obtained, the price is proper, the reaction condition is mild, and a novel and efficient new path is provided for the industrial production of the 2-chlorine-3-amino-4-picoline.
Owner:LUDONG UNIVERSITY +1
Synthesis method of 4-cyanopyridine
ActiveCN101602719ASimple processHigh yieldOrganic chemistryMetal/metal-oxides/metal-hydroxide catalysts4-MethylpyridineSynthesis methods
The invention discloses a synthesis method of 4-cyanopyridine, which is characterized in that 4-methylpyridine is vaporized and mixed with ammonia and air; the 4-methylpyridine reacts with the ammonia and air in the presence of a catalyst, and then the finished product of 4-cyanopyridine is obtained after absorption, extraction and rectification. The method features simple process and easy operation; the conversion rate of the 4-methylpyridine is more than 99% and the yield of the 4-cyanopyridine is more than 98%.
Owner:NANTONG ACETIC ACID CHEM
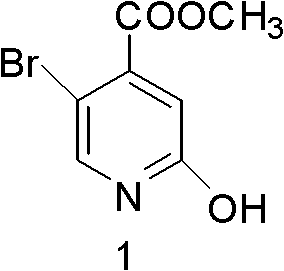
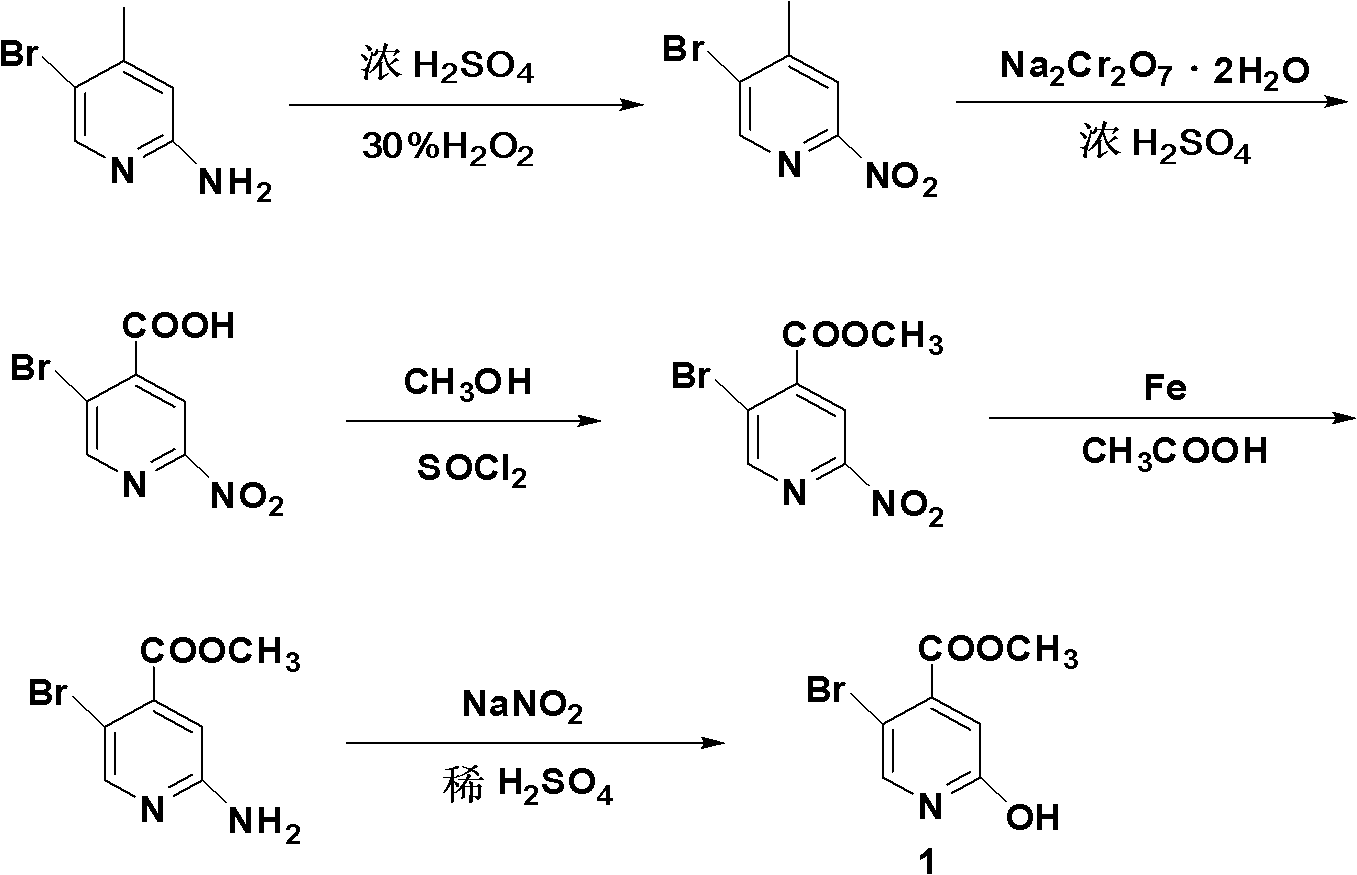
Synthesis method of 5-bromo-2-methyl 4-hydroxypyridinecarboxylate
InactiveCN102321016AHigh yieldRaw materials are easy to getOrganic chemistry4-MethylpyridineHydrolysis
The invention provides a synthesis method of 5-bromo-2-methyl 4-hydroxypyridinecarboxylate. 5-bromo-2-methyl 4-hydroxypyridinecarboxylate is prepared by five-step reaction namely two-step oxygen, esterification, reduction, diazotization hydrolysis and the like by taking 2-amino-5-bromo-4-methylpyridine as a raw material, wherein the total yield is more than 40%. According to the invention, the raw material is available, a used intermediate is cheap in price, reaction steps are simple and easy to operate, reaction process is safe and reliable, and the yield of the product is high, thus the synthesis method has potential industrial application value.
Owner:QILU UNIV OF TECH
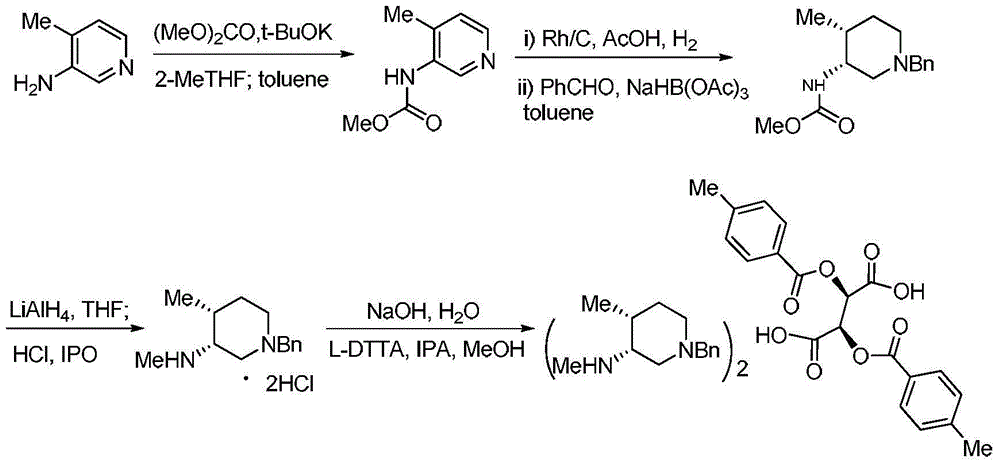
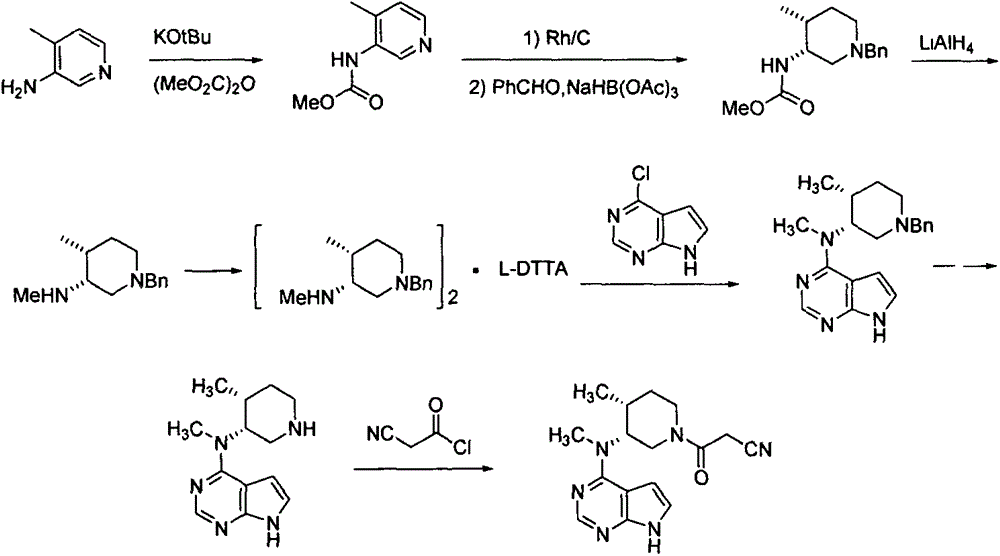
Bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate synthesis method
The present invention relates to a novel preparation method of a tofacitinib intermediate bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate. According to the preparation method, 3-amino-4-methyl piperidine is adopted as a starting raw material, is subjected to a N-methoxycarbonylation reaction under the sodium hydride effect, and is subjected to a catalytic hydrogenation reaction, a nucleophilic substitution reaction, an amide reduction reaction under the red aluminum effect, and L-di-p-toluyl tartaric acid (L-DTTA) chiral splitting so as to finally prepare the target compound bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate. The process of the present invention has characteristics of further existing process improving, simple operation, easy post-treatment, high yield, and low cost.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
High-yield high-purity DAST source powder synthetic process
The invention discloses a high-yield high-purity DAST source powder synthetic process. The synthetic process comprises the following two steps: 1, reacting 4-methylpyridine with methyl p-toluenesulfonate in the presence of absolute ethyl alcohol serving as a solvent to obtain an absolute ethyl alcohol solution of 4-methyl-N-methylpyridine tosilate; and 2, reacting 4-methyl-N-methylpyridine tosilate with p-dimethylaminobenzaldehyde in the presence of absolute ethyl alcohol serving as a solvent and under the catalytic action of di-n-butylamine or piperidine, so as to obtain high-yield (85-95%) high-quality (90-95%) DAST source powder. According to the synthetic process, absolute ethyl alcohol is used as a reaction solvent, harm of poisonous and harmful solvents such as methylbenzene and methyl alcohol to the body of an operator can be avoided and the pollution of waste liquid to the environment can be avoided. The research success of the high-yield high-purity DAST source powder synthetic process is beneficial to culture of high-quality large-sized DAST crystal, thereby laying a good material and theoretical basis for research on the DAST crystal and related products.
Owner:CHINA ELECTRONICS TECH GRP NO 46 RES INST
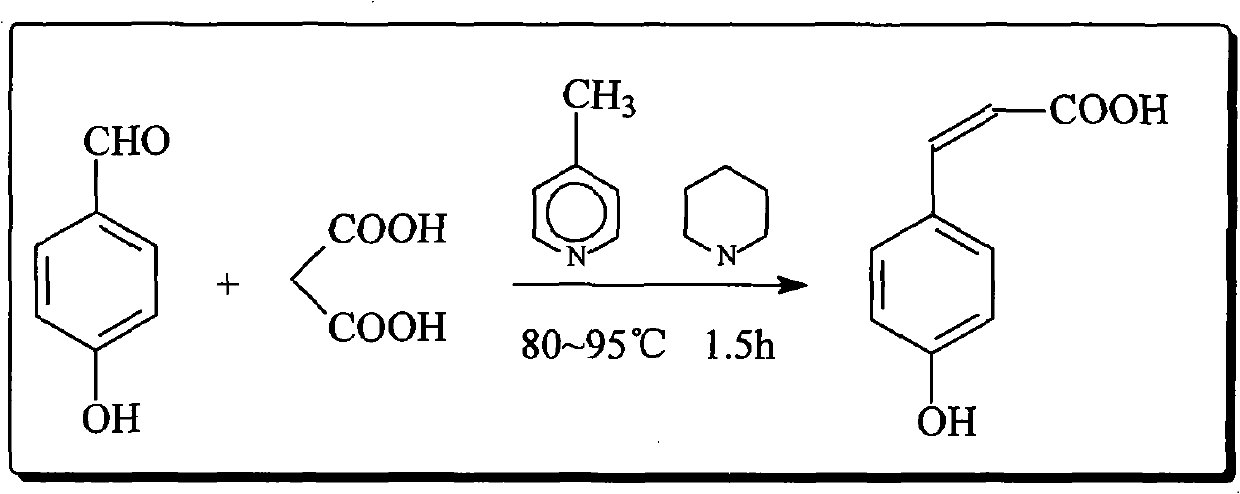
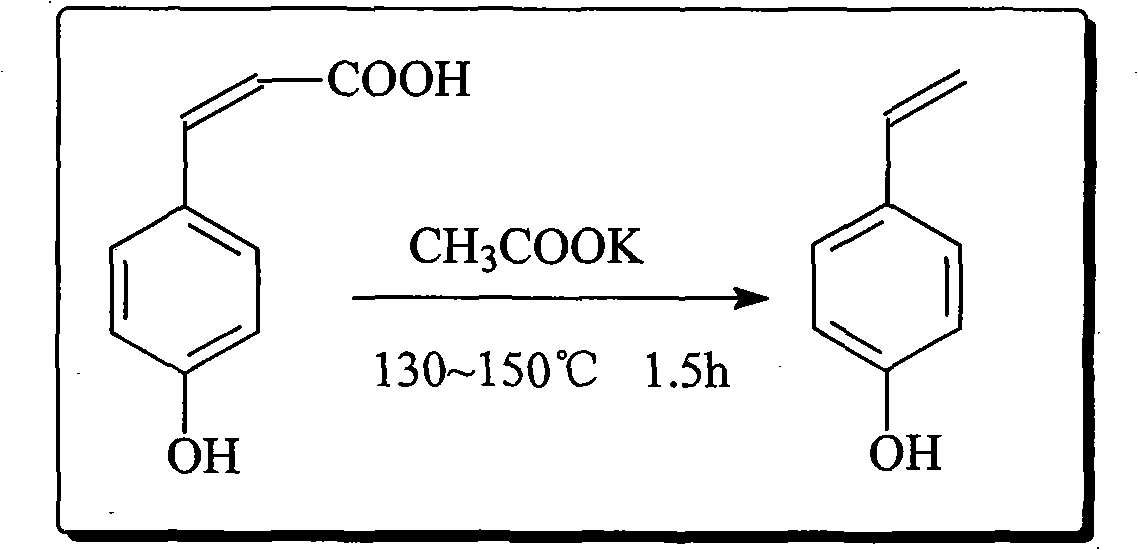
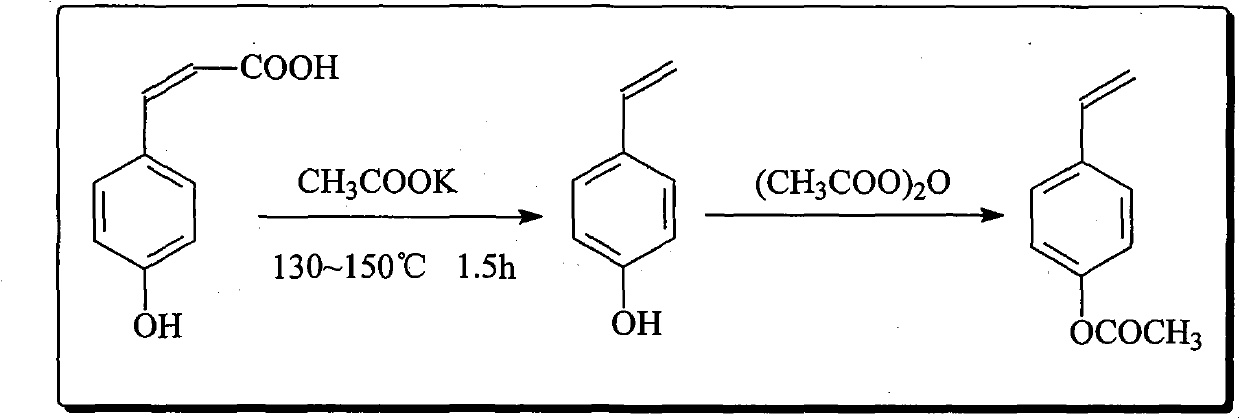
Preparation method of p-hydroxystyrene and derivates thereof
ActiveCN102001918AHigh yieldReduce odorOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalysts4-MethylpyridineOrganic synthesis
The invention relates to the field of organic synthesis, in particular to a preparation method of p-hydroxystyrene and derivates thereof. The preparation method is as follows: preparing p-hydroxycinnamic acid; preparing the p-hydroxystyrene; and finally preparing 4-vinyl phenyl acetate or 4-tert-butoxycarbonyloxystyrene. In the preparation process of the p-hydroxycinnamic acid, 4-picoline replaces pyridine to serve as a reaction catalyst, the yield of the p-hydroxycinnamic acid is improved to about 95%, and the problems of odor of pyridine and low recovery rate are alleviated. Cyclohexane is used as an extraction solvent to extract the p-hydroxystyrene derivate from a reaction liquid, and the residue of the solvent in the product is greatly reduced. The traditional synthetic route of the 4-tert-butoxycarbonyloxystyrene is changed, and the Witting reaction with rigorous conditions is avoided; and meanwhile the complex separation and purification problems such as column chromatography are simplified, and the yield is about 80% which is higher than that of current reports.
Owner:五莲县计量检测服务中心
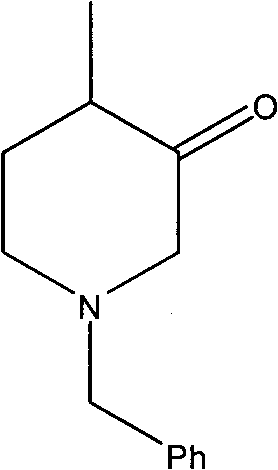
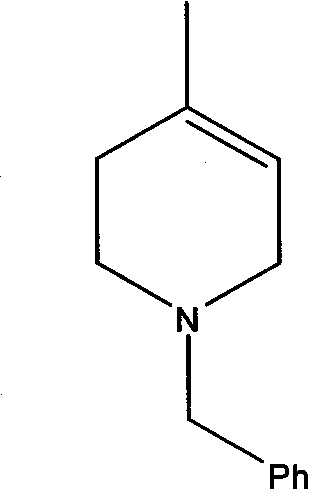
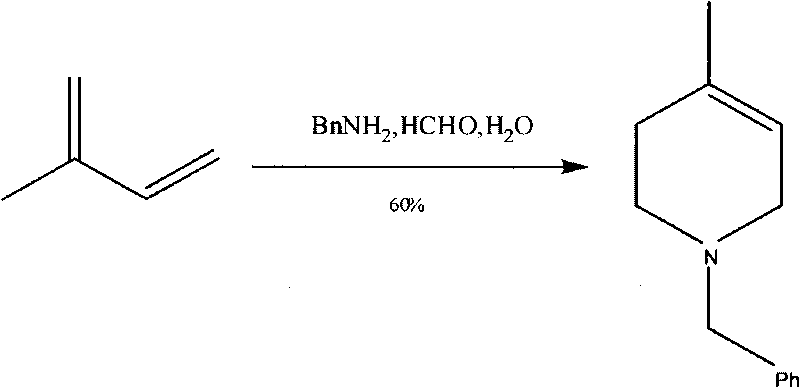
Method for synthesizing N-benzyl-4-methyl-3-piperidone
The invention discloses a method for synthesizing N-benzyl-4-methyl-3-piperidone, which takes 3-hydroxy-4-methyl-pyridine and benzyl chloride as initial raw materials. The method comprises the following steps of: (1) adding the 3-hydroxy-4-methyl-pyridine into a reaction solvent I, dropwise adding the mixed liquid of the benzyl chloride and the reaction solvent I at room temperature and heating until refluxing; (2) adding the benzyl chloride salt of the 3-hydroxy-4-methyl-pyridine obtained in the step (1) and a reducer into an alkaline water solution and then heating until refluxing; and (3) adding N-benzyl-3-hydroxy-4-methyl piperidine obtained in the step (2) into a reaction solvent II, dropwise adding a CrO3 solution and concentrated sulfuric acid and reacting at room temperature to obtain the N-benzyl-4-methyl-3-piperidone. The synthesizing method has the characteristics of high total yield and the like.
Owner:ZHEJIANG UNIV
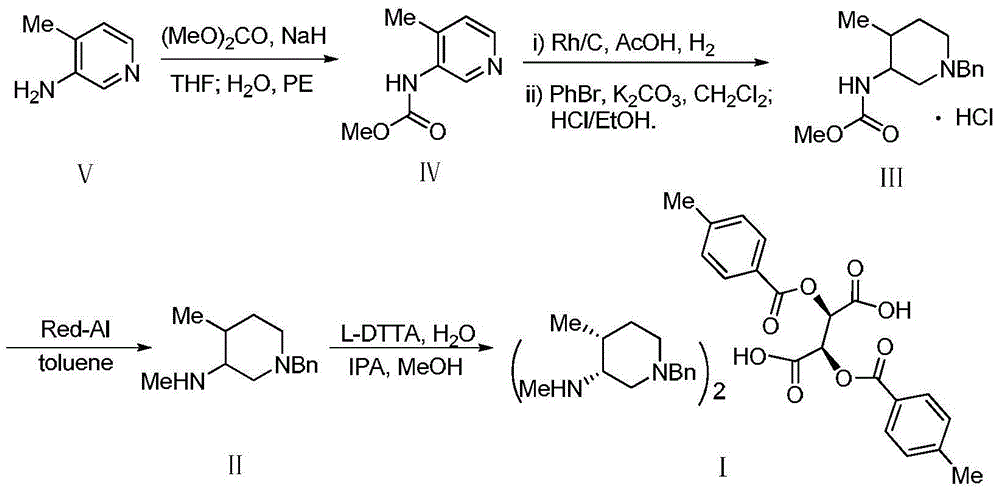
Preparation method of tofacitinib citrate starting material
InactiveCN107337676AMeet the needs of industrial productionReduce manufacturing costOrganic chemistryPyridiniumSynthesis methods
The invention discloses a synthesis method of a tofacitinib citrate starting material N-((3R, 4R)-4-methyl-1-benzyl-3-piperidyl)-N-methyl-7-tolylsulfonyl-7H-pyrrolo[2,3-D]pyrimidine-4-amine(I). The method comprises the specific steps that 4-methylpyridine is adopted as the starting material to be subjected to nucleophilic substitution with benzyl chloride to obtain 4-methyl-1-benzyl-pyridinium chloride, a reduction reaction is performed under the effect of sodium borohydride, a hydroboration-oxidation reaction is performed, hydroxyl oxidation is performed, two chiral centers are introduced in a reductive amination stereoselectivity mode, splitting is performed through cheap chiral acid (L-DTTA) easy to obtain to obtain an optically pure intermediate body (3R, 4R)-(1-benzyl-4-methyl-piperidine-3-yl)-methyl amine, and finally, the intermediate body and 4-chloropyr are condensed to obtain the tofacitinib citrate starting material. The whole method is easy to implement and low in cost, raw materials are easy to obtain, and aftertreatment is easy. The formula is shown in the description.
Owner:JIANGSU QINGJIANG PHARMA
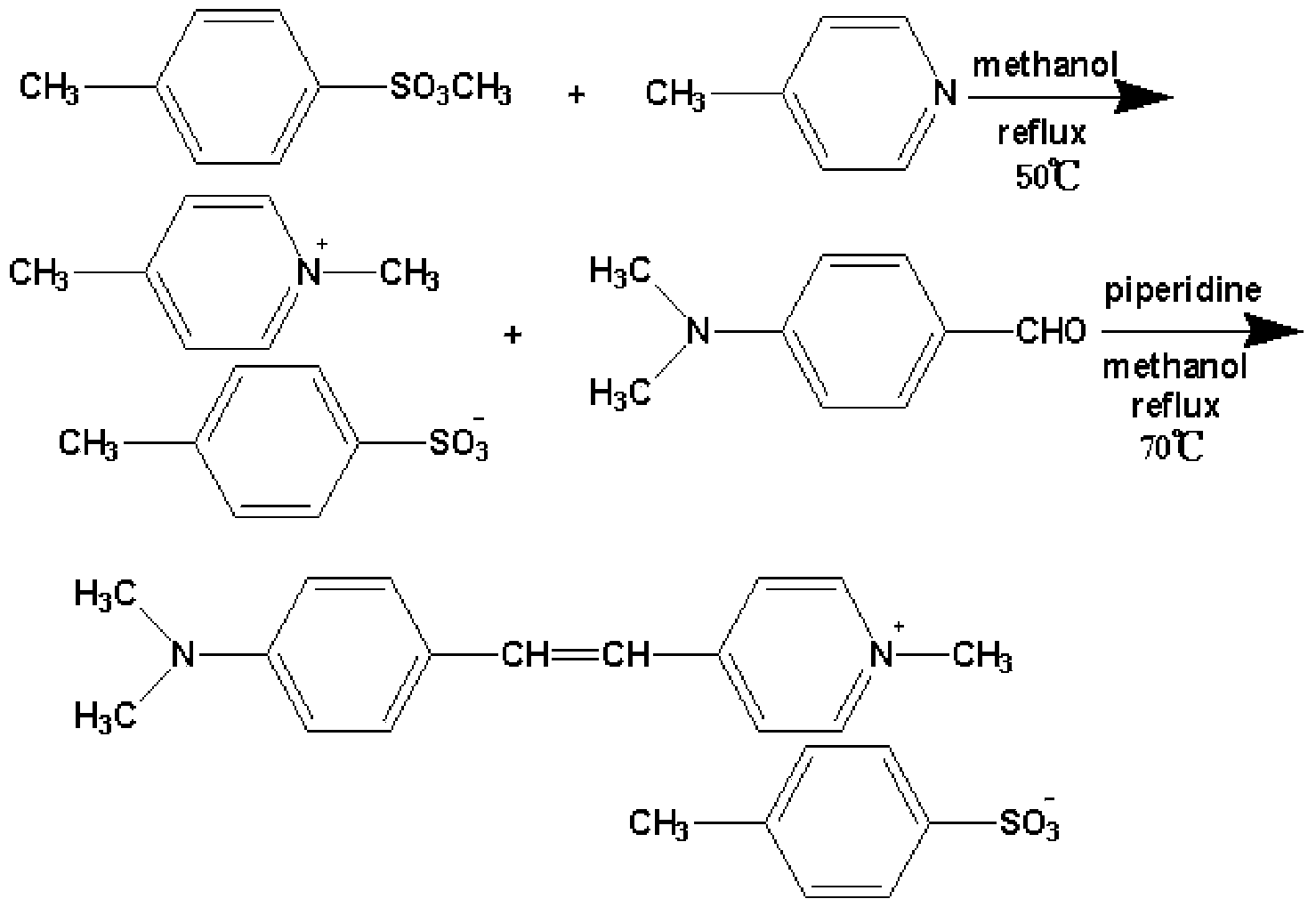
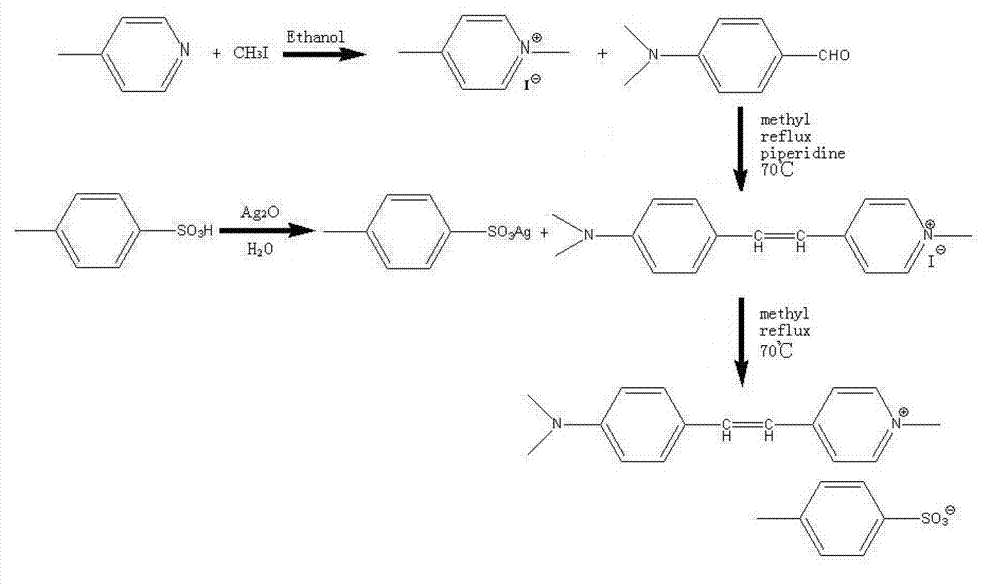
Preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate
InactiveCN103467366AEasy to prepareThe reaction steps are simpleSulfonic acids salts preparationBenzaldehydeIon exchange
The invention belongs to the technical field of functional material synthesis, and relates to a preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate, which comprises the following steps: mixing 4-methylpyridine and methyl-p-toluene sulfonate, putting in absolute methanol, and pouring into a three-hole flask to carry out ion-exchange reaction; dissolving dimethyl amino benzaldehyde in absolute methanol, and putting into the three-hole flask to carry out condensation reflux reaction to obtain a crystallized product; and flushing the crystallized product with trichloromethane, quickly filtering to obtain a solid with green metal luster, heating the solid with green metal luster to completely dissolve the solid in the absolute methanol, cooling, airing, and recrystallizing to obtain the 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate. The preparation method has the advantages of simpleness, simple reaction steps, short reaction time, low cost and high yield.
Owner:QINGDAO UNIV
Synthesis method for tofacitinib
The invention relates to a synthesis method for tofacitinib serving as a JAK inhibitor. According to the method, the tofacitinib is prepared by taking (4-picoline-3-yl)methyl carbamate as a raw material, and performing catalytic hydrogenation, benzyl protection, reduction, salification, separation, deprotection and amidation salification. The method specifically comprises the following steps: (1) performing catalytic hydrogenation and reduction on (4-picoline-3-yl)methyl carbamate in sulfuric acid and Pd / C; (2) reacting cis-(4-picoline-3-yl)methyl carbamate and benzyl chloride to obtain cis-(1-benzyl-4-methylpiperidine-3-yl)methyl carbamate; (3) performing HOBT catalytic condensation on N-[(3R,4R)-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine and cyanoacetic acid to obtain tofacitinib free alkali. The preparation method provided by the invention has easily obtained raw materials, mild reaction conditions, easiness and convenience in operation and high yield, and is suitable for industrial production.
Owner:济南扬诺生物科技有限公司
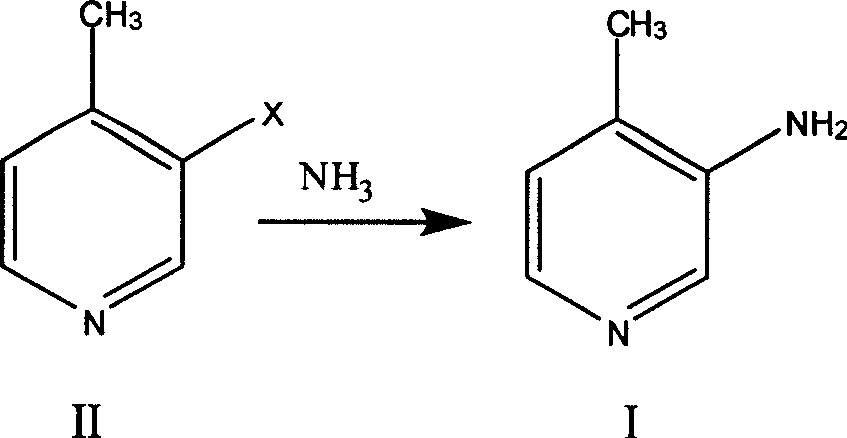
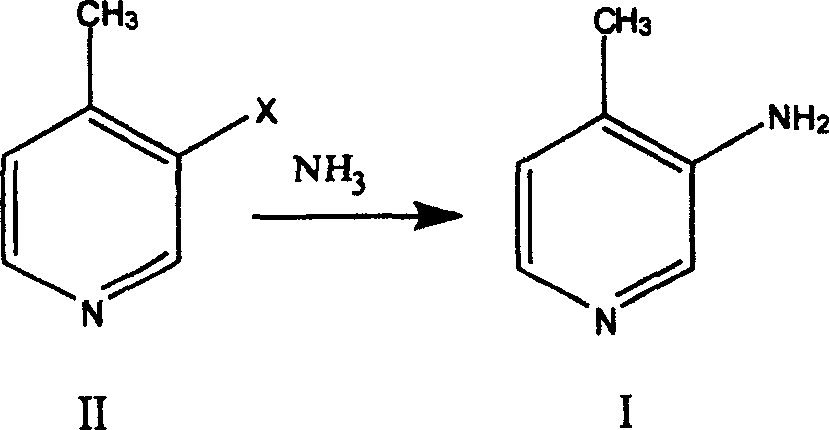
Preparation process of 3-amino-4 methyl pyridine
InactiveCN100460394CReduce manufacturing costHigh yieldOrganic chemistry4-MethylpyridineAnti aids drug
This invention relates to the field of pharmaceutical chemistry, involves the preparation method of Intermediate 3-amino-4-methylpyridine (1) of a specific anti-AIDS drugs nevirapine. The feature is it prepared by the 3-halogenated-4-methylpyridine, the preparation method is simple, mild conditions, and the yield is high.
Owner:CHINA PHARM UNIV +1
Method for recycling silver ion from sewer sludge by using novel chelate resin
InactiveCN105294890AWide variety of sourcesHigh mechanical strengthOther chemical processesProcess efficiency improvementN dimethylformamideSynthesis methods
The invention discloses a method for recycling silver ions from sewer sludge by using novel chelate resin. The synthesis method of chelate resin comprises the following steps: 1), soaking micrococcus chlorine into N,N-dimethylformamide as a reaction solvent till the micrococcus chlorine is sufficiently swelled; 2) adding 2-amino-6-methylpyridine as ligand and sodium as a catalyst into a product obtained in the step 1), in the presence of nitrogen, stirring at 80 DEGC to react for 10 hours, wherein the amount ratio of 2-amino-4-methylpyridine to -CH2Cl in micrococcus chlorine is 4:1; and 3) filtering the product obtained in the step 2), thereby obtaining a filtrate cake, firstly, soaking the filtrate cake in a reaction solvent, washing until a washing liquid is colorless, washing with distilled water, washing for times with absolute ethyl alcohol, acetone, diethyl ether and distilled water, and performing vacuum drying at 50 DEG C, thereby obtaining novel chelate resin. The method has the characteristics of good selective adsorption of silver ions in sewer sludge, good adsorption property, cyclic utilization, and the like.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
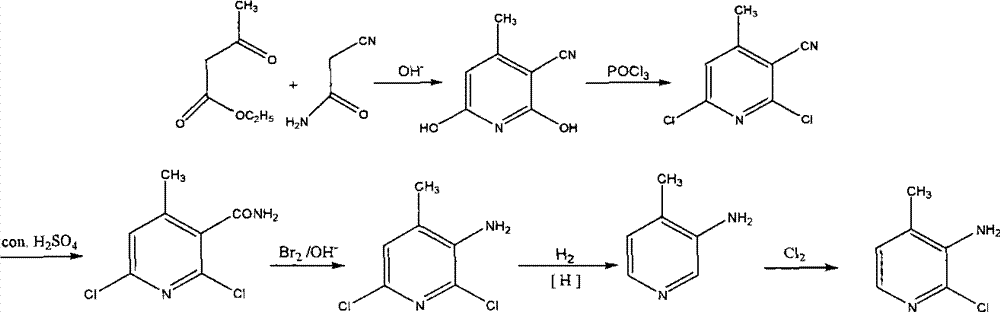
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399AComplicated operationFew stepsOrganic chemistrySodium methoxideOrganic synthesis
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing the reaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
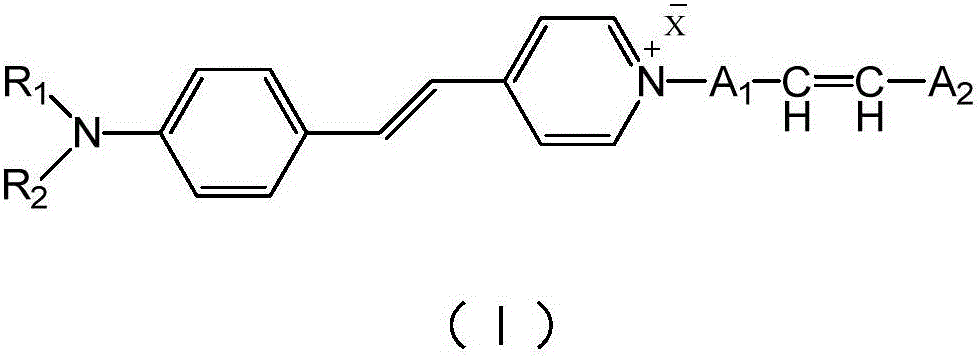
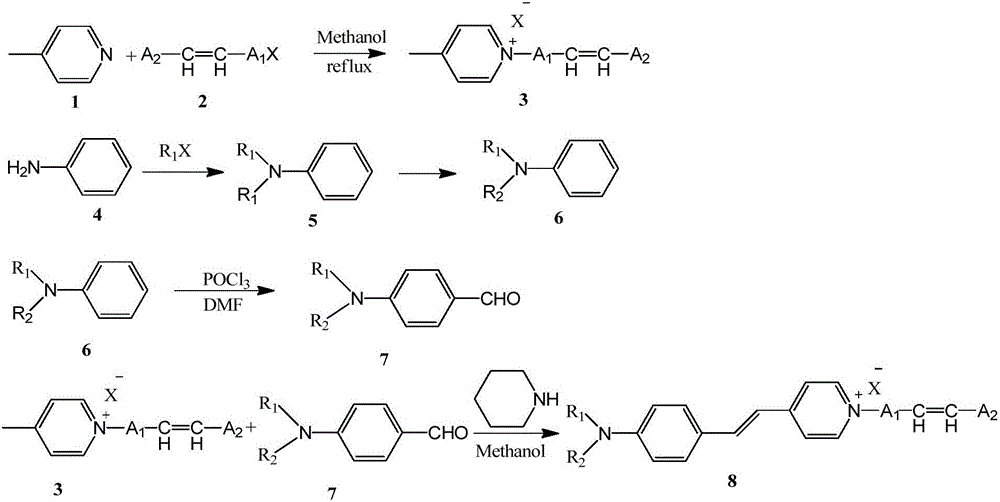
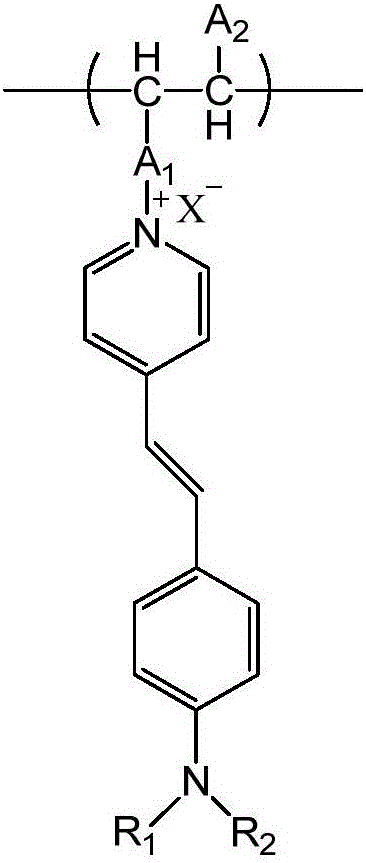
Aminostyrylpyridinium-salt fluorescent monomer and preparing and application of polymer of aminostyrylpyridinium-salt fluorescent monomer
The invention relates to an aminostyrylpyridinium-salt fluorescent monomer, a synthesis method of a polymer of the aminostyrylpyridinium-salt fluorescent monomer and application prospects of the water-soluble fluorescent polymer in the field of oil fields. The fluorescent monomer structure is shown as the formula (I) (please see the specification). The synthesis method includes the steps that 4-methylpyridine and alkyl halide are subjected to an alkylation reaction to generate 4-methylpyridine salt, aniline is subjected to a N-alkylation reaction or an acylation reaction and then subjected to a Vilsmeier reaction, an aldehyde group is led in a para-position mode, and finally, the aldehyde group and methyl of the 4-methylpyridine salt are condensated to generate the aminostyrylpyridinium-salt fluorescent monomer. The fluorescent monomer and water-soluble monomers such as acrylamide, acrylic acid, methacrylamide, methylpropanesulfonic acid, N,N-dimethylacrylamide and N,N-diethylacrylamide are subjected to binary polymerization or terpoly polymerization, and a water-soluble fluorescent tracing polymer is prepared. The fluorescent tracing polymer can be applied to concentration detection of the polymer through polymer flooding.
Owner:SOUTHWEST PETROLEUM UNIV
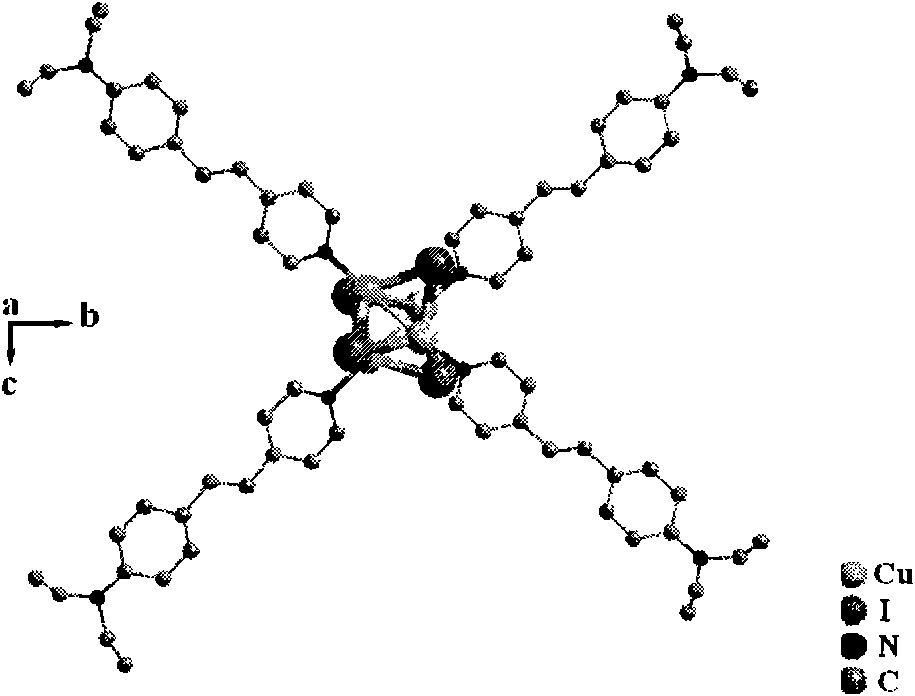
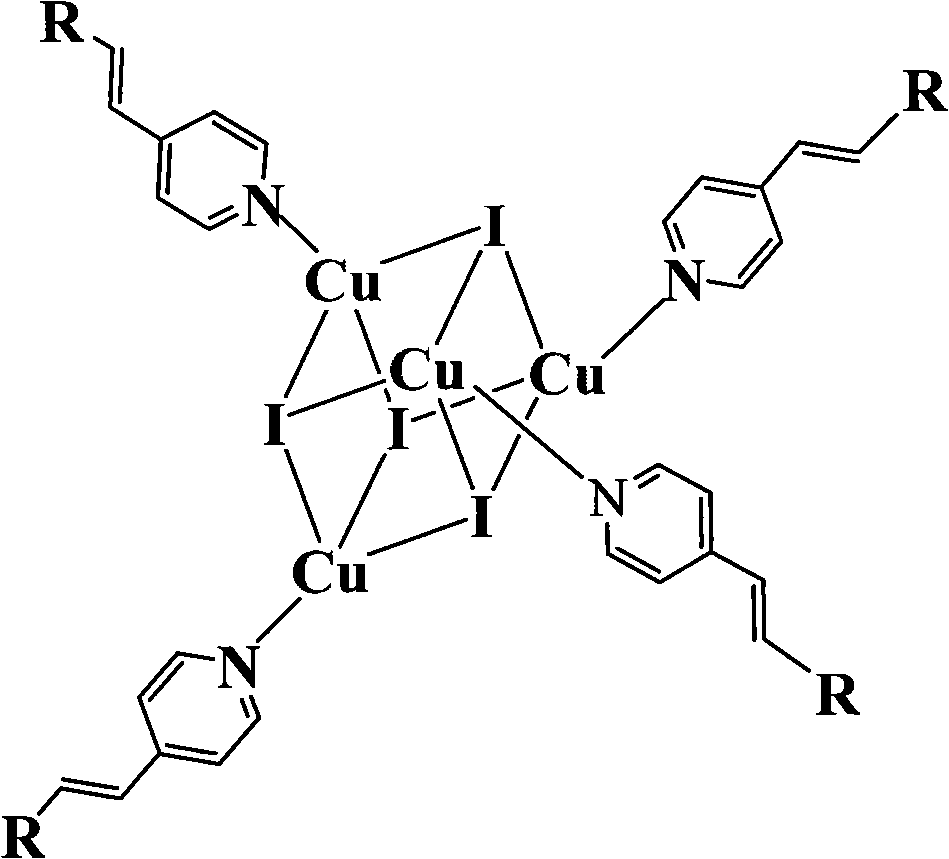
Copper cluster two-photon absorbing material with living cell developing function and synthetic method thereof
InactiveCN101787041AEasy to manufactureLow biological toxicityCopper organic compoundsLuminescent compositions4-MethylpyridineTwo-photon absorption
The invention provides a copper cluster two-photon absorbing material with a living cell developing function, which is a univalent copper cluster with a multi-branched general chemical formula. The copper cluster two-photon absorbing material is prepared by the steps of: firstly, preparing a pyridine ligand from 4-methylpyridine and 4-N,N-2R-aminobenzaldehyde; and secondly, synthesizing a target product by using the pyridine ligand and cuprous iodide.
Owner:ANHUI UNIVERSITY
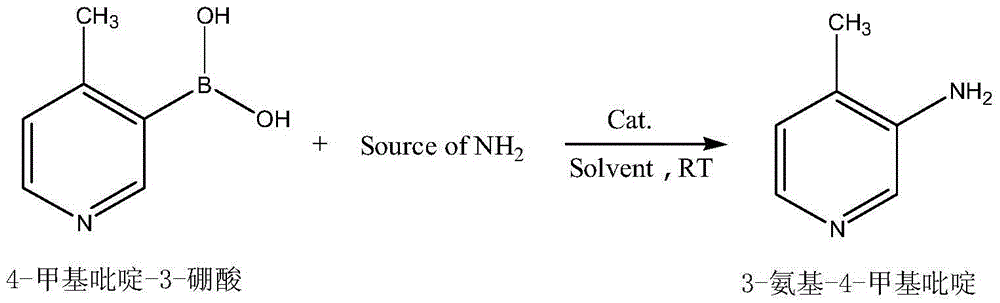
Preparation method of 3-amino-4-methylpyridine
ActiveCN104356057AThe reaction process is simpleHigh yieldOrganic chemistryChemical synthesisSynthesis methods
The invention belongs to the field of organic chemical synthesis and particularly relates to a synthesis method of 3-amino-4-methylpyridine which is an intermediate of an anti-AIDS drug nevirapine. According to the method, 4-methylpyridine-3-boronic acid is used as a raw material, an inorganic amide is used as an ammonia source and obtain3-amino-4-methylpyridine is prepared through one-step reaction in the presence of metal oxide as a catalyst. The preparation method is still simple and provides a new way for novel and efficient synthesis of 3-amino-4-methylpyridine and the disadvantages of long traditional route, low yield and severe reaction conditions are overcome.
Owner:JIANGSU ZHONGBANG PHARMA
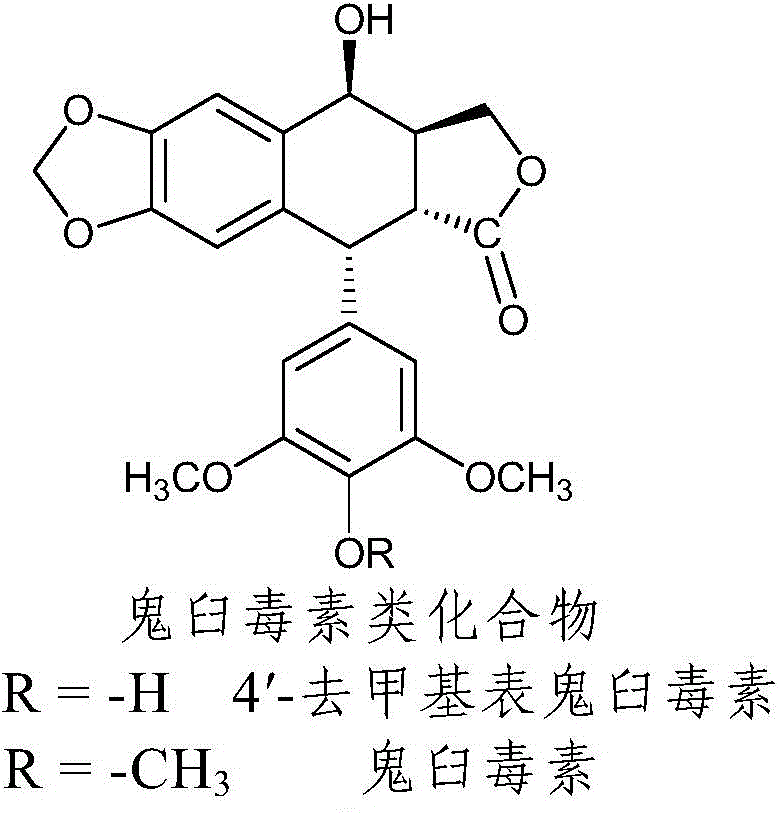
Nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and preparation method and use thereof
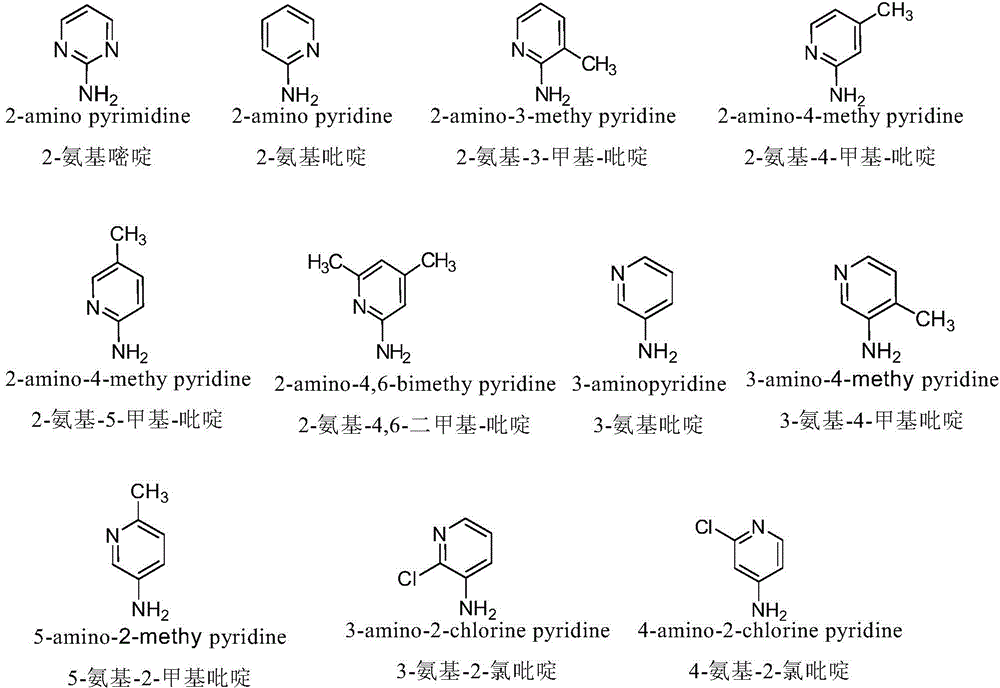
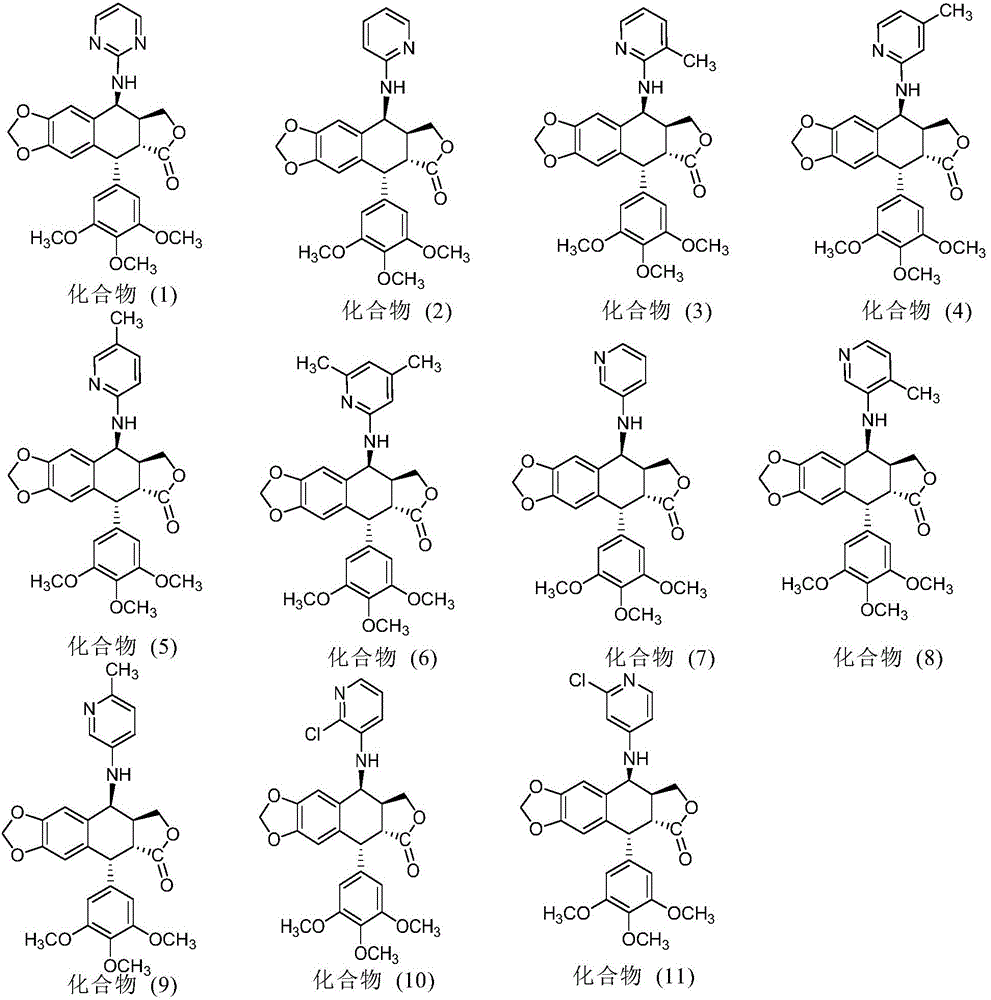
InactiveCN103601732AGood antitumor activityImprove anti-tumor activityOrganic active ingredientsOrganic chemistryTumor cells2-Methylpyridine
The invention discloses a nitrogen-substituted podophyllotoxin derivative with anti-tumor activity and a preparation method and use thereof. According to the method, 2-aminopyrimidine, 2-aminopyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, 2-amino-5-methylpyridine, 2-amino-4,6-dimethyl pyridine, 3-aminopyridine, 3-amino-4-methylpyridine, 5-amino-2-methylpyridine, 3-amino-2-chloropyridine or 4-amino-2-chloropyridine is respectively introduced to an activated C-ring fourth position of a podophyllotoxin compound through nitrogen substitution reaction, so as to obtain the nitrogen-substituted podophyllotoxin derivative, represented by a formula (V) shown in the specification, with excellent anti-tumor activity. The nitrogen-substituted podophyllotoxin derivative disclosed by the invention acts on tumor cells through multiple ways and multiple target points, and the anti-tumor activity of the nitrogen-substituted podophyllotoxin derivative is remarkably improved compared with that of the podophyllotoxin compound. The compound disclosed by the invention can be used for preparing anti-tumor drugs and is clinically applied to anti-tumor treatment.
Owner:HUBEI UNIV OF TECH
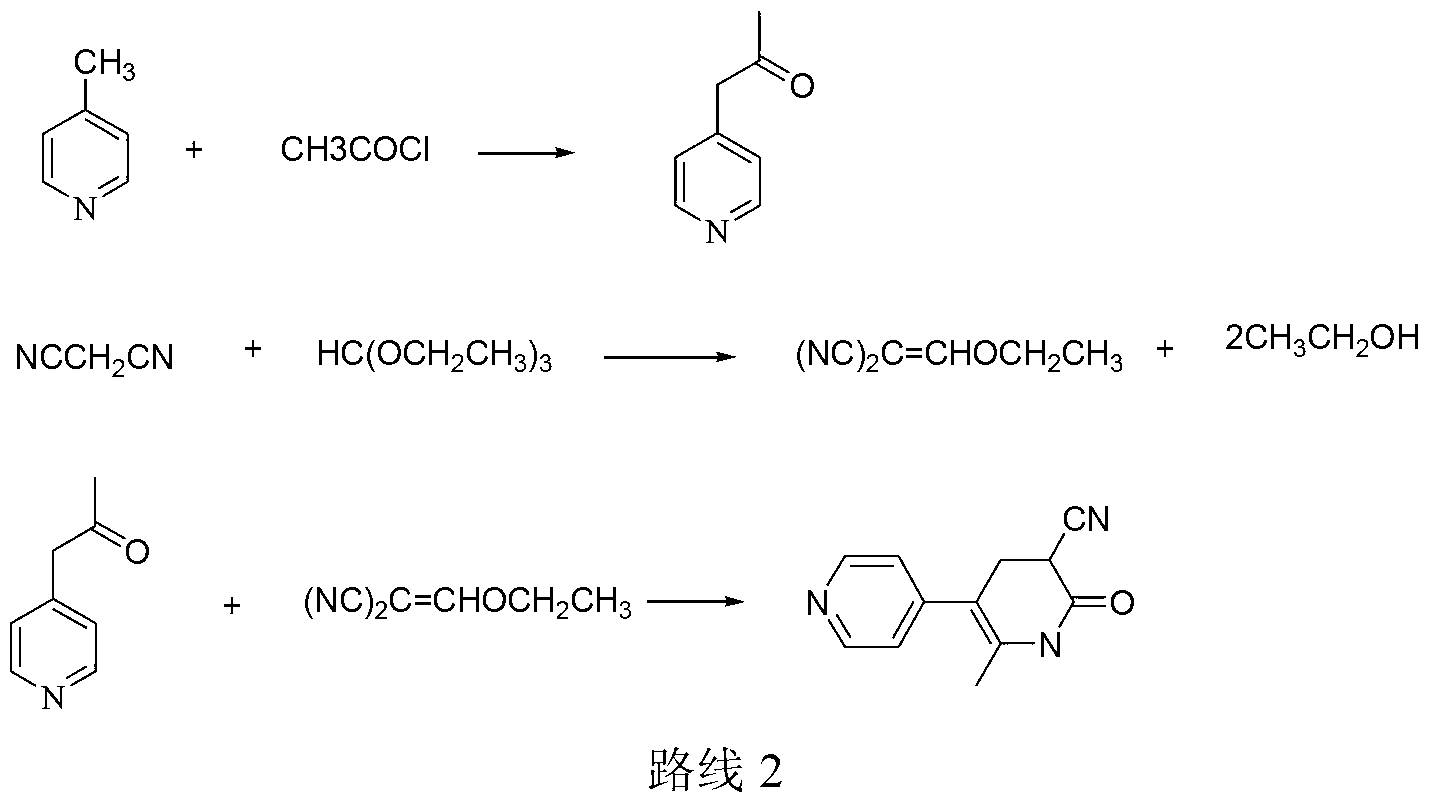
Synthesis method of milirinone
InactiveCN103804288AEasy to operateReduce pollutionOrganic chemistryChemical industry4-Methylpyridine
Belonging to the fields of chemical industry and chemical medicine, the invention discloses a synthesis method of milirinone. The method includes: adopting 4-methylpyridine as a raw material to undergo acetylation reaction with acetic anhydride, subjecting the acetylate to condensation with triethyl orthoformate directly without purification, and subjecting the condensation product and malononitrile to cyclization reaction in ethanol absolute, thus obtaining milirinone. The method is characterized by starting with relatively simple and easily available raw materials to directly obtain a complicated structure molecule without separation and purification of the intermediates. The milirinone synthesized by the method provided by the invention has the advantages of high yield and high sample purity. Milirinone is an important chemical product, and can be used as a cardiotonic drug and the like.
Owner:SICHUAN XINSIDUN PHARMA
High performance liquid chromatographic detection method for 3-cyanopyridine and 4-methylpyridine in 4-cyanopyridine
The invention discloses a high performance liquid chromatographic detection method for 3-cyanopyridine and 4-methylpyridine in 4-cyanopyridine. The high performance liquid chromatographic detection method comprises the following steps: (1) preparing a system applicable solution; (2) preparing a test solution; 3) preparing a contrast solution. The system applicable solution, the test solution and the contrast solution are detected respectively by adopting high performance liquid chromatography. The detection condition is as follows: octadecylsilane chemically bonded silica is used as a filling agent of a chromatographic column; a mobile phase A is modified buffer salt, and is prepared by adding 20 mmol of potassium dihydrogen phosphate and 2.4 ml of triethylamine into 1000 ml of water and adjusting the pH value to 6.0-8.0 with phosphoric acid; a mobile phase B is an organic phase, and adopts gradient elution; the flow rate is 0.5-1.5 ml / min; the column temperature is 10-40 DEG C; the detection wave length is 200-300 nm; the injected sample volume is 10 [mu]l. The separation degree of 3-cyanopyridine, 4-cyanopyridine and 4-methylpyridine in the detection method are all 2.0 or above, the theoretical plate number is high, the symmetry is good, and the condition that the accuracy of the detection results is influenced by interference between the components is effectively avoided; meanwhile, the detection method has the advantages of being accurate and reliable in detection result, short in analysis time, simple and convenient to operate, and the like.
Owner:江苏悦兴医药技术有限公司
Preparation method of fluorescent probe for identification of microfilament bacteria
ActiveCN104017570AHigh fluorescence intensityFluorescent properties are stableMethine/polymethine dyesOrganic chemistry4-MethylpyridineCyanine
The invention provides a preparation method of a fluorescent probe for identification of microfilament bacteria. The method first uses halogenated long carbon chain compound to modify 4-methyl pyridine part of a carbazole pyridine styrene cyanine dye, and then the dye reacts with 3-formyl-N-ethyl carbazole for the synthesis of the fluorescent probe with a long hydrophobic chain. The invention has the beneficial effect that modification and preparation of the carbazole pyridine styrene cyanine dye are synchronized in the probe preparation process, the preparation method is simple, and the prepared fluorescent probe has little background interference, high fluorescence intensity and stable fluorescent property. The characteristic of hydrophobic surface of microfilament bacteria is utilized that the prepared fluorescent probe with long hydrophobic chain in this application can combined with microfilament bacteria to achieve the purpose of fluorescent identification of microfilament bacteria.
Owner:TIANJIN CHENGJIAN UNIV
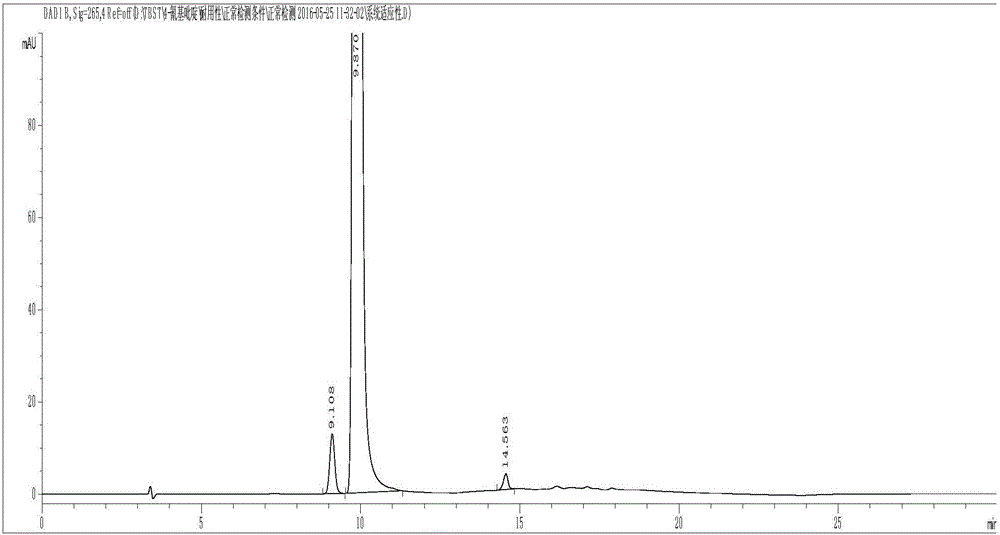
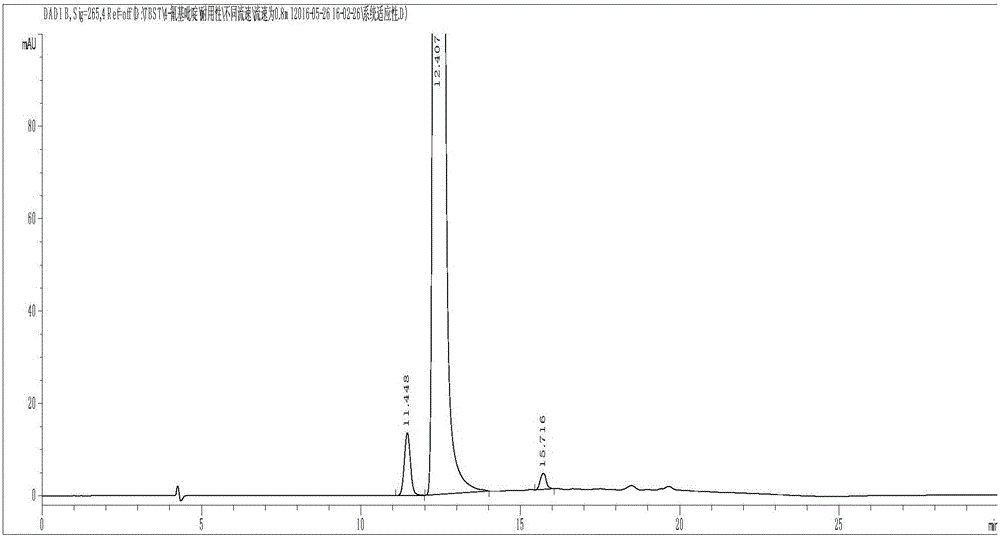
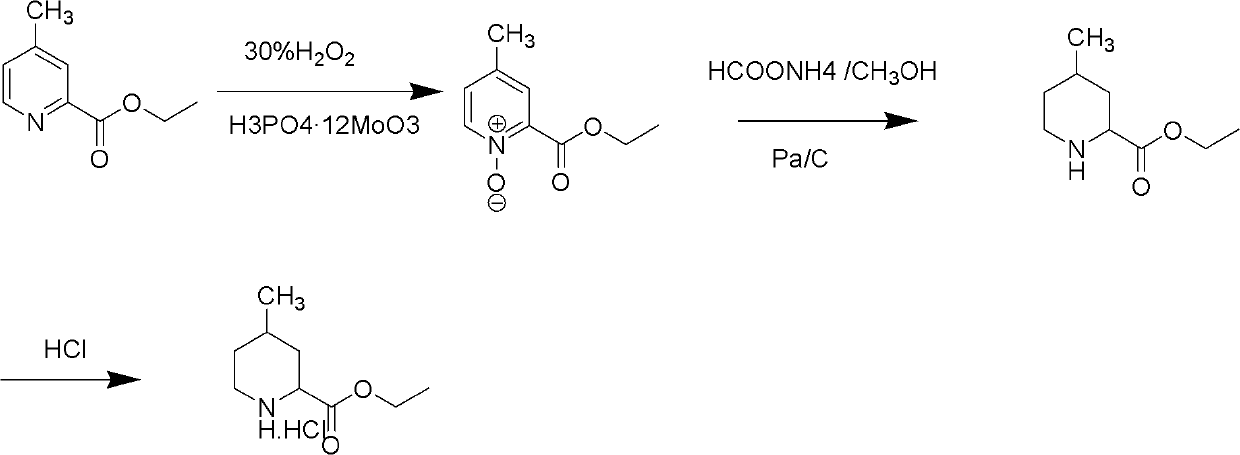
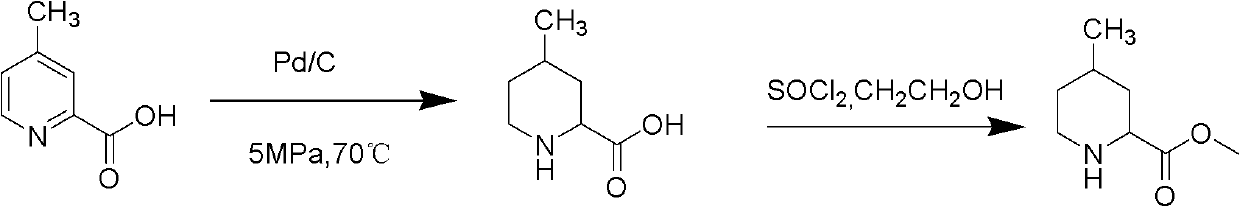
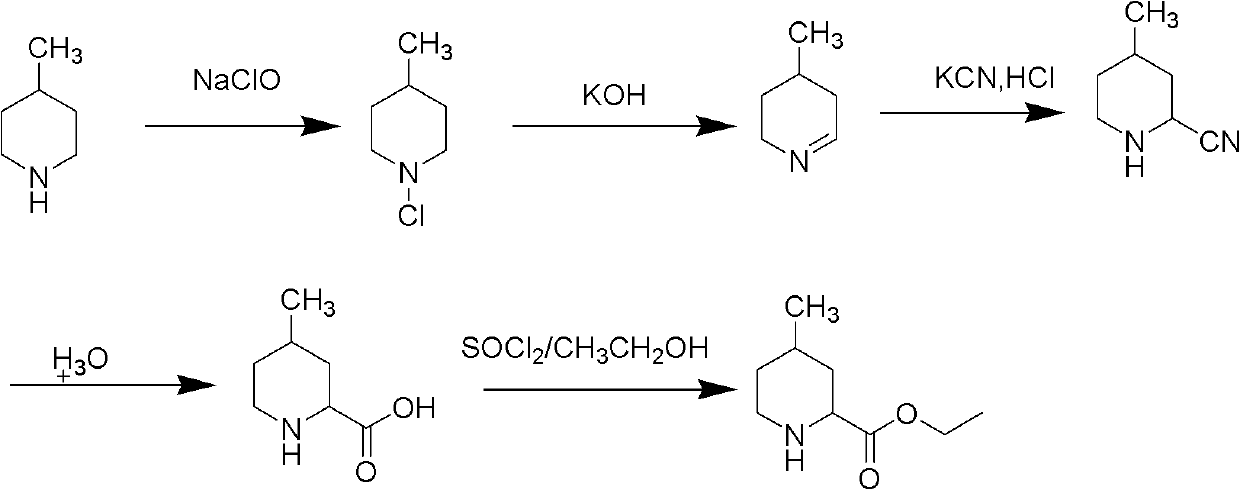
Method for preparing 4-methylpiperidine-2-carboxylate hydrochloride
ActiveCN102887854AEasy to operateMild conditionsOrganic chemistryPhosphomolybdic acid4-methylpiperidine
The invention relates to a method for preparing 4-methylpiperidine-2-carboxylate hydrochloride which is an intermediate of argatroban active precursor (2R, 4R)-4-methylpiperidine-2-carboxylic acid ethyl ester. 4-methylpiperidine-2-carboxylic acid ethyl ester is adopted as a raw material, phosphomolybdic acid is used as a catalyst, oxidizing is performed to obtain 4-methylpiperidine-2-carboxylic acid ethyl ester nitrogen oxide, methanol or ethanol is taken as a solvent, and 4-methylpiperidine-2-carboxylate hydrochloride is produced through reduction reaction. The method is simple to operate and has moderate conditions; phosphomolybdic acid is adopted to increase the activity of an oxidizing agent of hydrogen peroxide, the usage of the oxidizing agent is reduced, and the reaction yield is increased; the purification is convenient, the product quality is excellent, and good safety is obtained; and pollution is reduced, and industrial production is benefited.
Owner:CHANGSHAN BIOCHEM PHARM JIANGSU CO LTD
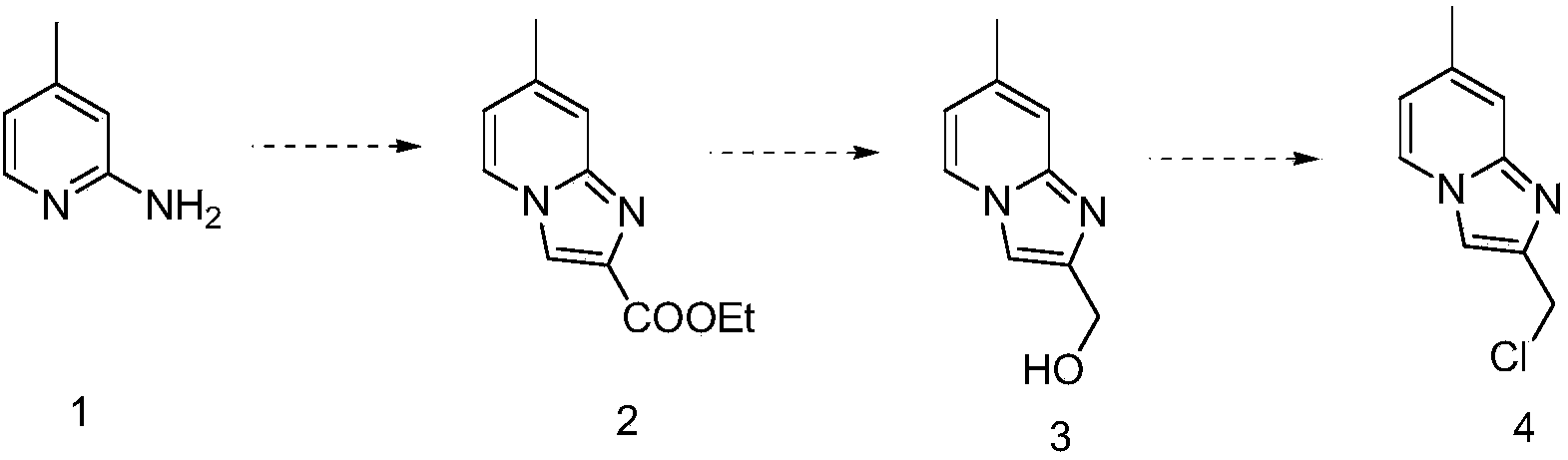
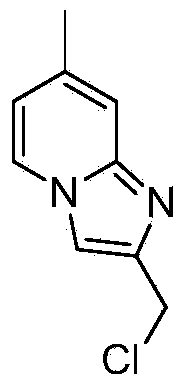
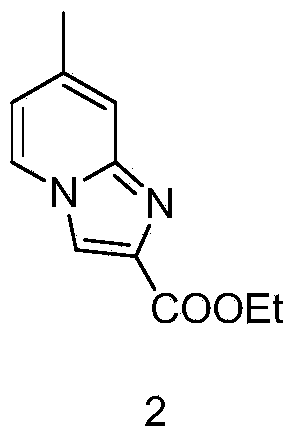
Preparation method of imidazopyridine derivative
The invention discloses a preparation method of imidazopyridine derivative 2-(chloromethyl)-7-methyl-imidazo-[1,2-a] pyridine. A target product is obtained through ring closing, reduction and chlorination based on 2-amido-4-methylpyridine as an initial raw material. The compound is an important medical intermediate.
Owner:湖南华腾制药有限公司
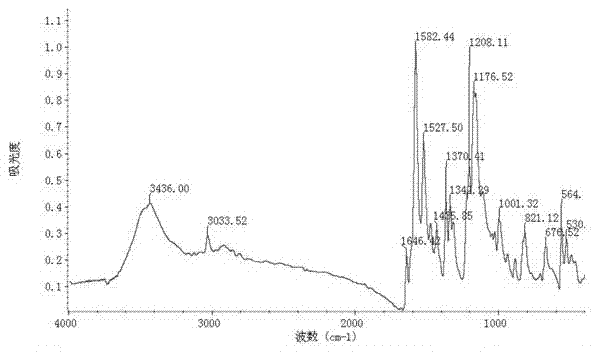
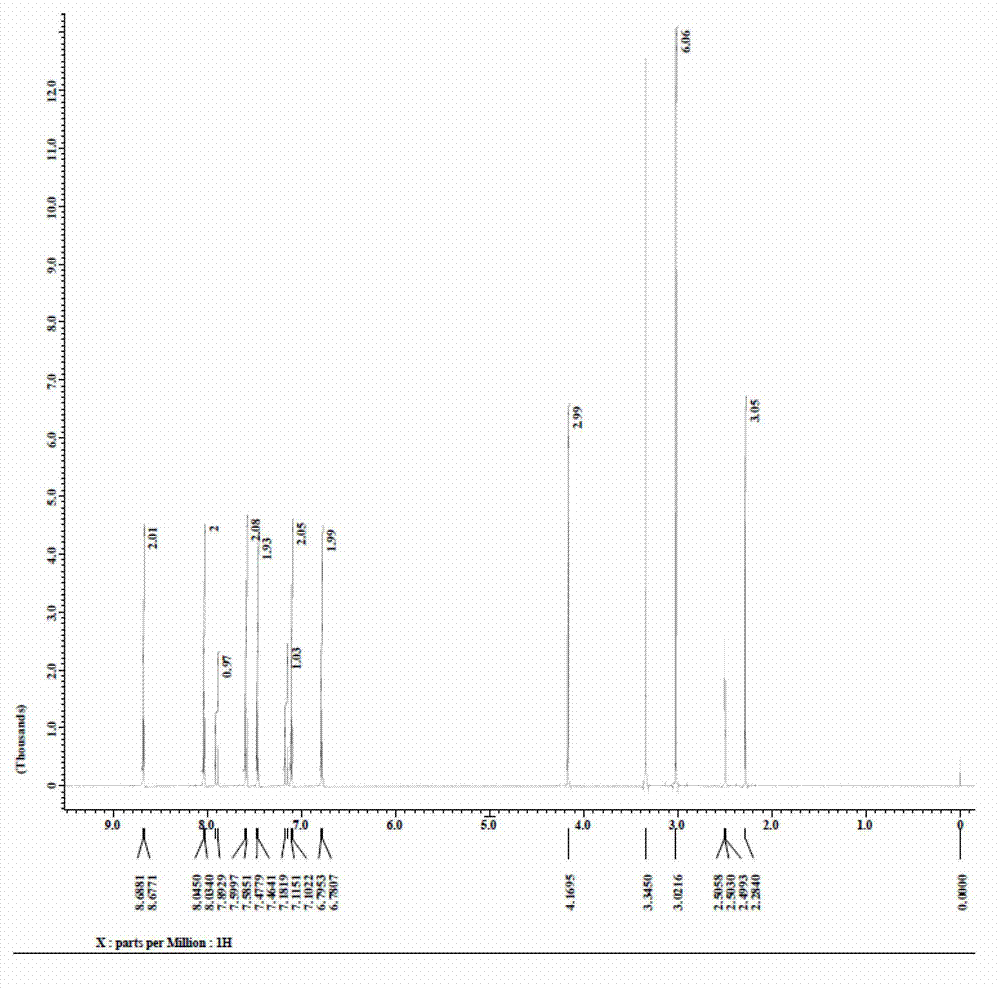
Preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate
The invention belongs to the technical field of functional material preparation, and relates to a preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate, which comprises the steps of synthetic reaction, condensation reaction, silver p-toluenesulfonate, exchange reaction and purification by filtration. The method specifically comprises the following steps: dissolving 4-methylpyridine and iodomethane in ethanol to carry out reaction, thereby generating 4-methylpyridinium iodide; dissolving 4-methylpyridinium iodide and p-dimethylaminobenzaldehyde in methanol, and adding a catalyst to react, thereby generating 4-(4-dimethylaminostyryl)methylpyridyl iodide; reacting p-toluenesulfonic acid and silver oxide to generate silver p-toluenesulfonate; reacting the silver p-toluenesulfonate and 4-(4-dimethylaminostyryl)methylpyridyl iodide to generate silver iodide and 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate; and purifying by filtration. The invention has the advantages of simple preparation technique, reliable principle, low preparation cost, high product purity, mild reaction conditions, high yield and environment friendliness.
Owner:QINGDAO UNIV
Eco-friendly process for the preparation of 2-Chlorobenzylidene-Malononitrile (CS)
ActiveUS7732631B2Reduce sewage loadSpeed up the processCarboxylic acid nitrile preparationOrganic compound preparation4-MethylpyridineMorpholine
An improved process for the preparation of 2-chlorobenzylidenemalononitrile (CS) comprising of the steps of: preparing malononitrile suspension by adding 5-20% (wt %) preferably 12-14% malononitrile to water while constantly stirring and then adding 0.05-0.5% (v / v) preferably 0.1-0% of a catalyst like piperidine, pyridine, 2-picoline, 3-picoline, 4-picoline or morpholine preferably piperidine piperidine with constant stirring at 20-30° C.; condensing the malononitrile suspension prepared in step (a) with 2-chlorobenzaldehyde by adding 10-15% (w / v) preferably 25-30%, of 2-chlorobenzaldehyde cover a period at 30-45 minutes so that the temperature of the reaction mixture remains below 50° C., constantly stirring for 20-40 minutes, then filtering the CS and drying it at 20-30° C. under water vacuum for 3-5 hrs.
Owner:DIRECTOR GENERAL DEFENCE RES & DEV ORG
Method for preparing nevirapine
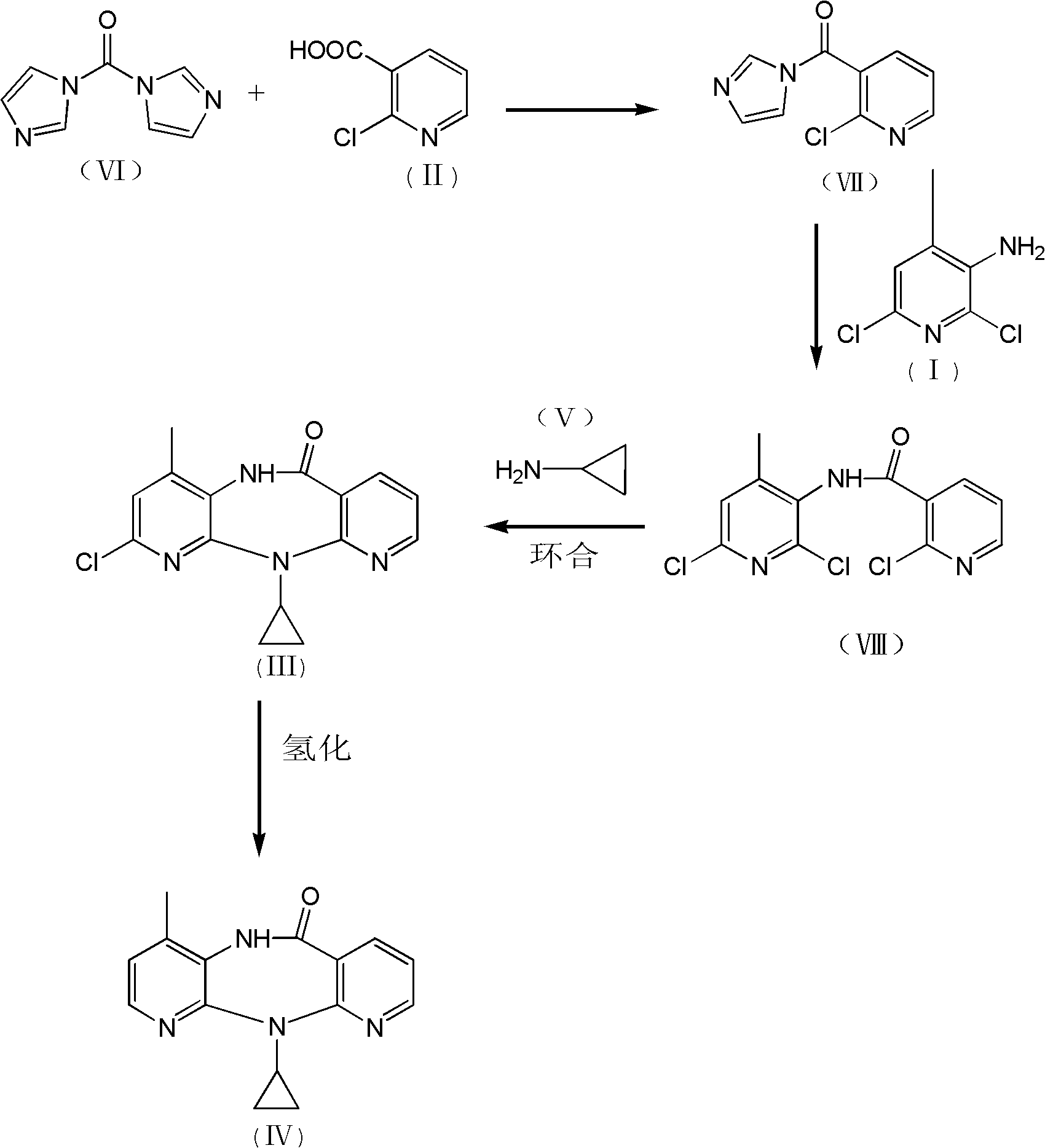
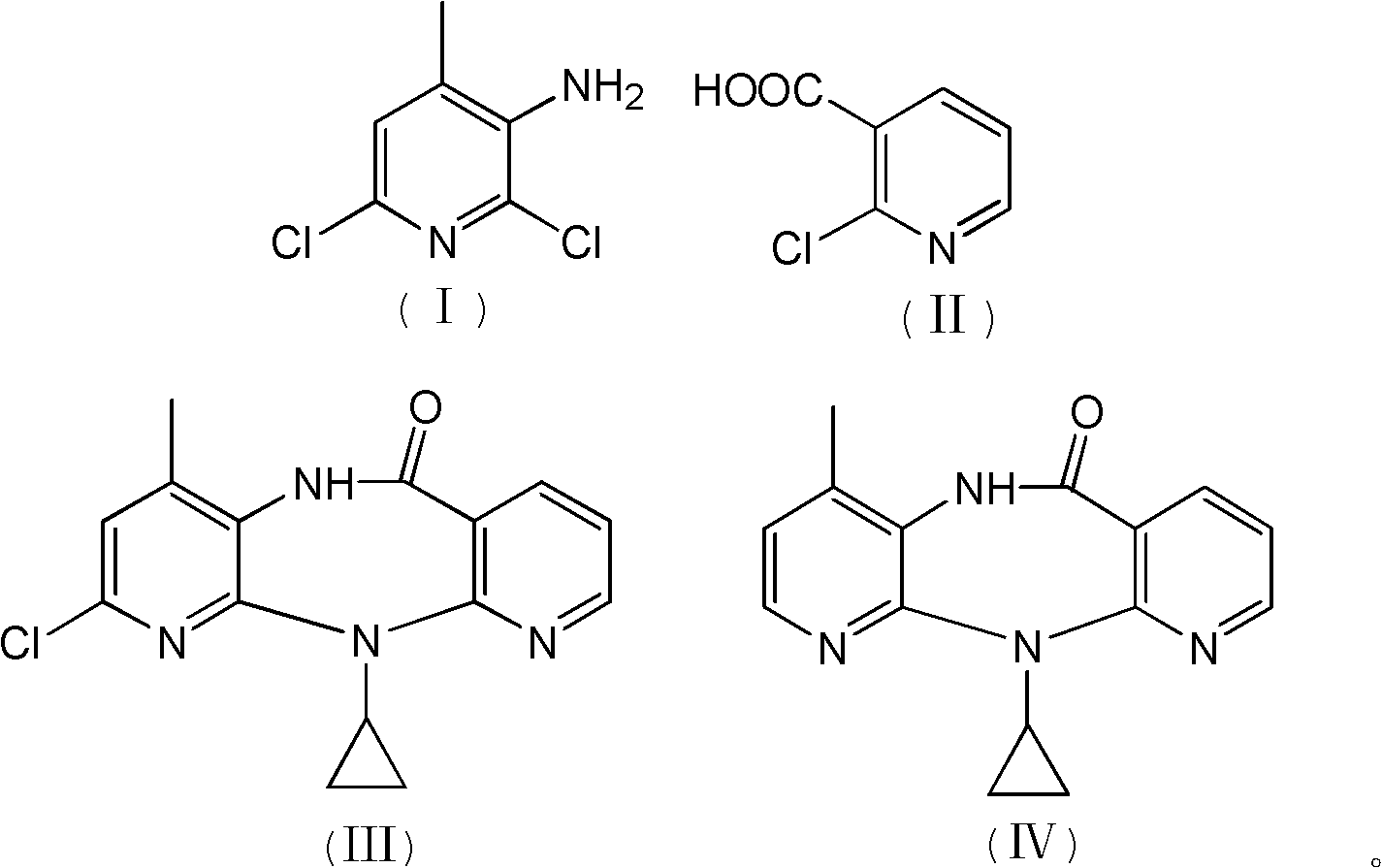
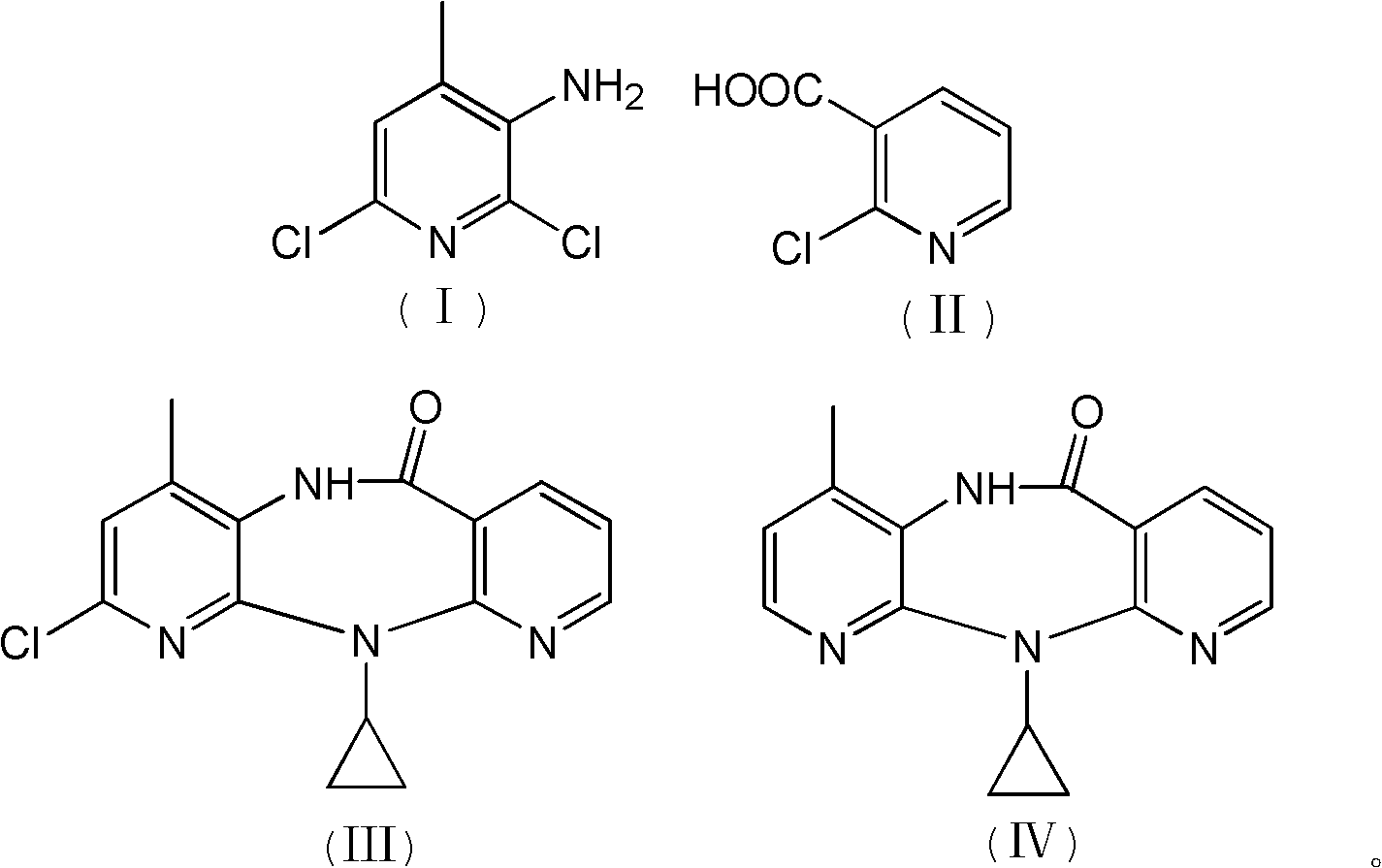
InactiveCN102127077AAvoid pollutionAvoid Adding Air Scrubbing EquipmentOrganic chemistryAntiviralsNiacinamideCarboxylic acid
The invention discloses a method for preparing nevirapine, which comprises the steps of: A. enabling 2-chlorine-3 picolinic acid shown in the structural formula (II) and carbonyl diimidazole to react to produce active amide; B. enabling the active amide produced in the step A to react with 2, 6- dichloro-3-amino-4-methylpyridine shown in the structural formula (I), and producing N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide; C. carrying out nucleophilic substitution reaction on the N-(2, 6-dichloro-4-methyl-3-pyridyl)-2-chlorine niacinamide obtained in the step B and cyclopropylamine, then carrying out cyclization on the obtained products to obtain 2-chloro nevirapine with structure shown in the formula (III); and D. under the presence of catalyst, carrying out hydrogenation reaction on the 2-chloro nevirapine with structure shown in the formula (III) in the step C and non-hydrogen hydrogen source, to obtain the nevirapine with structure shown in the formula (IV). The invention aims at providing a method for preparing the nevirapine, which is low in cost, environment-friendly, higher in safety and suitable for production.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method for preparing 2-aminoisonicotinic acid
The invention discloses a method for preparing 2-aminoisonicotinic acid. 2-amino-4-methylpyridine is used as a raw material. The method comprises the following steps of: protecting amino in acetic acid by acetylation of acetyl chloride, oxidizing methyl in potassium permanganate under the action of a catalyst, performing hydrolysis by using sodium hydroxide, and performing neutralization by using concentrated hydrochloric acid to obtain the 2-aminoisonicotinic acid. The raw materials used in the method are cheap, particularly the yield is improved after the catalyst is added, the utilization rate of raw materials is increased, and the method has good economic benefit.
Owner:GUANGDONG UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com