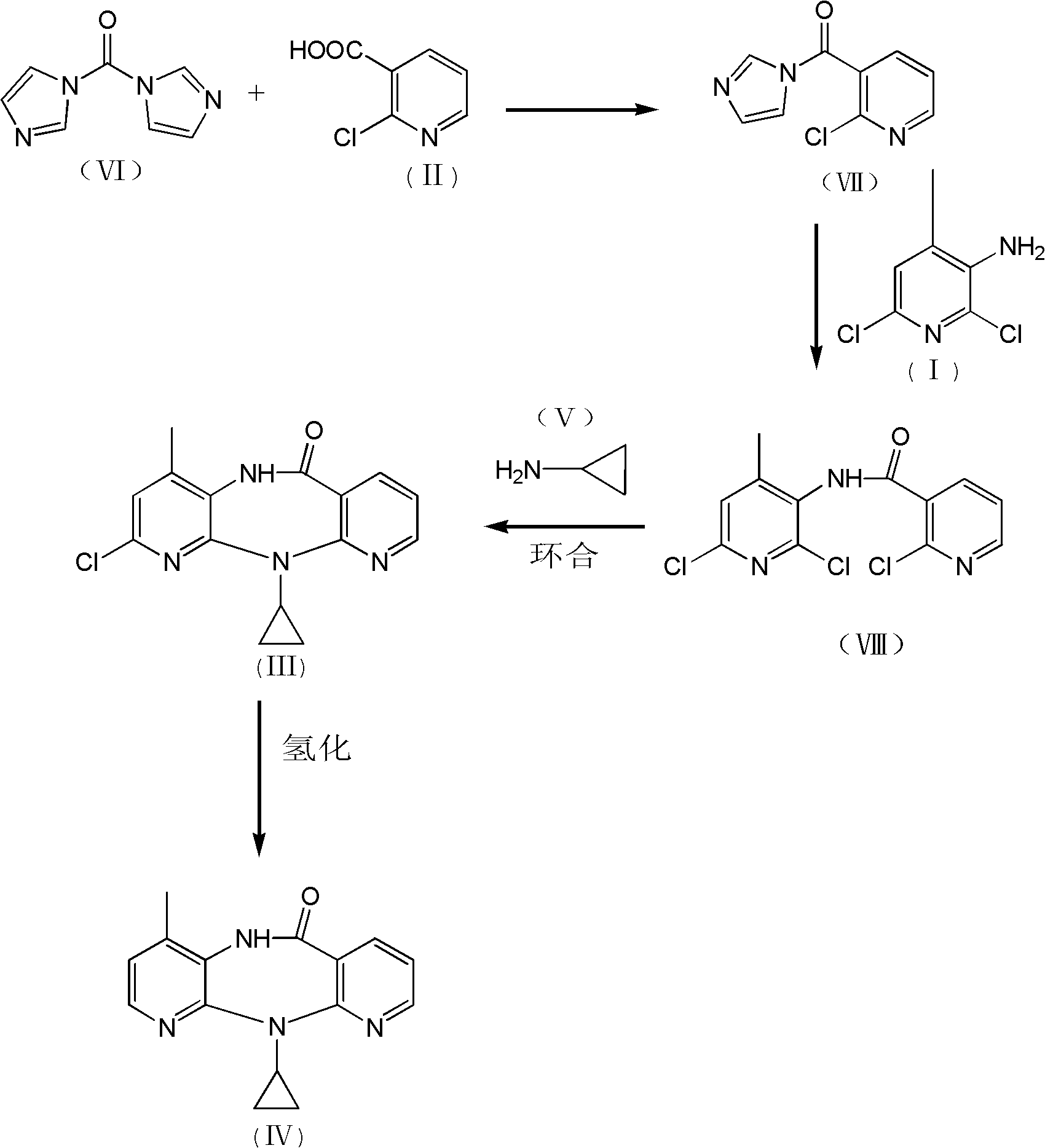
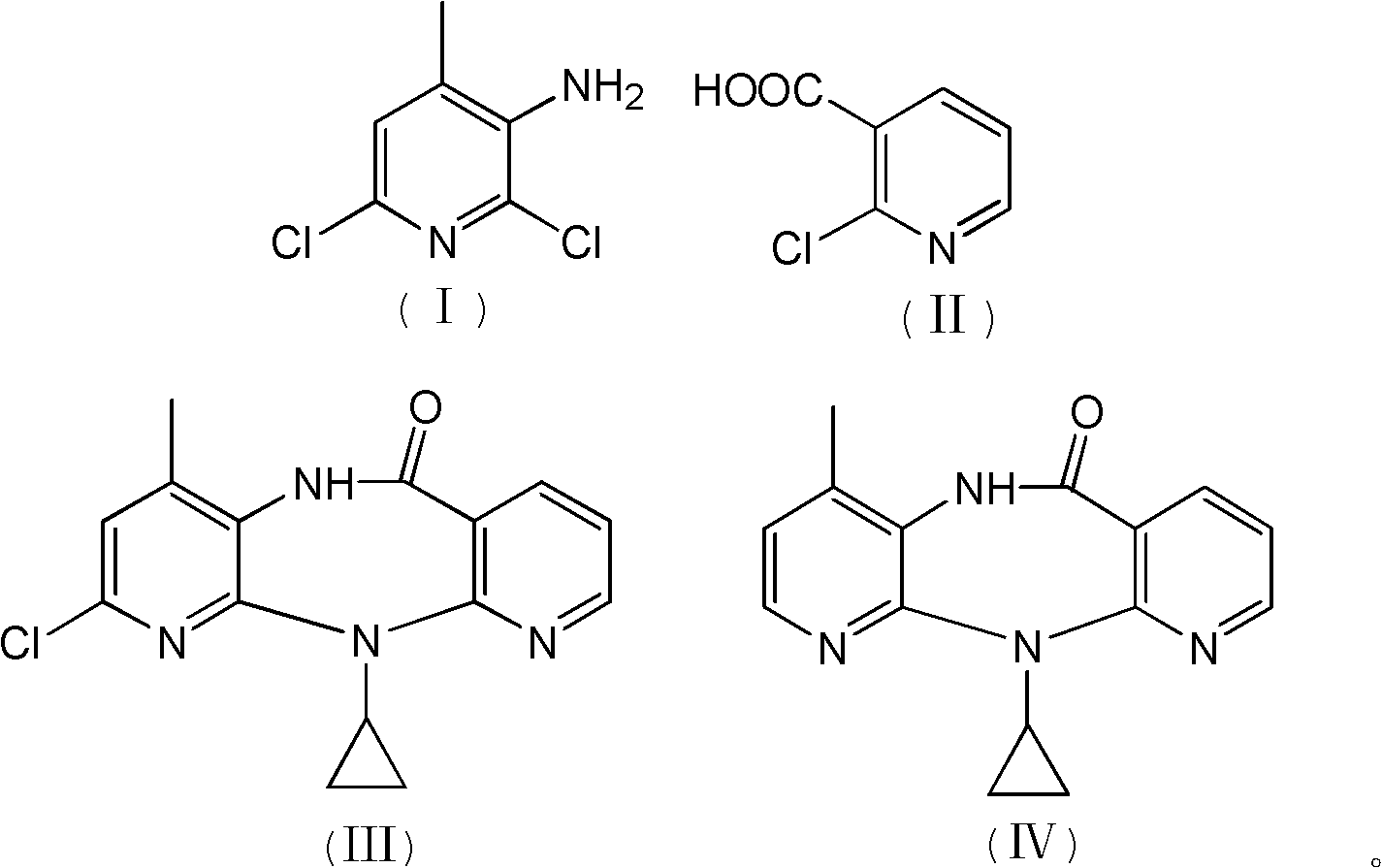
Method for preparing nevirapine
A nevirapine and reaction technology, applied in the field of preparation of nevirapine, can solve the problems of only 73% yield, danger, low hydrodechlorination efficiency, etc., and achieve the effects of simple post-processing, reduced risk, and reduced equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Preparation of N-(2,6-dichloro-4-methyl-3-pyridyl)-2-chloronicotinamide:
[0033] Add 120.0g of 2-chloro-3-pyridinecarboxylic acid, 650mL of toluene, and 0.5mL of 4-picoline into a 1000mL three-necked flask, and stir at room temperature for 15 minutes under nitrogen protection; cool down to -25°C, and add carbonyldiimidazole in portions 123.6g, during which the temperature was controlled at -20°C to -25°C. After the addition was completed, the reaction was maintained at -20°C to -25°C for 45 minutes; 116.0g of 2,6-dichloro-3-amino-4-methylpyridine was dissolved In 750mL of dry toluene, then drop into the active amide solution in the previous step, maintain -15°C to -20°C during the period, and react at -15°C to -20°C for 2 hours after the addition; after TLC checks that the reaction is complete, add 500mL Stirring with water, the solid precipitated out and was filtered. The solid was washed with 100mL water and 100mL toluene respectively. After vacuum drying, 170.0...
Embodiment 2
[0043] 1. Preparation of N-(2,6-dichloro-4-methyl-3-pyridyl)-2-chloronicotinamide:
[0044] Add 115.0g of 2-chloro-3-pyridinecarboxylic acid, 650mL of toluene, and 0.5mL of 4-picoline into a 1000mL three-necked flask, and stir at room temperature for 15 minutes under nitrogen protection; cool down to -25°C, and add carbonyldiimidazole in portions 118.4g, during which the temperature was controlled at -20°C to -25°C. After the addition was completed, the reaction was maintained at -20°C to -25°C for 45 minutes; 116.0g of 2,6-dichloro-3-amino-4-methylpyridine was dissolved In 750mL of dry toluene, then drop into the active amide solution in the previous step, maintain -15°C to -20°C during the period, and react at -15°C to -20°C for 2 hours after the addition; after TLC checks that the reaction is complete, add 500mL Stirring with water, the solid precipitated out and was filtered. The solid was washed with 100mL water and 100mL toluene respectively. After vacuum drying, 169.2...
Embodiment 3
[0054] 1. Preparation of N-(2,6-dichloro-4-methyl-3-pyridyl)-2-chloronicotinamide:
[0055] Add 125.0g of 2-chloro-3-pyridinecarboxylic acid, 650mL of toluene, and 0.5mL of 4-picoline into a 1000mL three-necked flask, and stir at room temperature for 15 minutes under nitrogen protection; cool down to -25°C, and add carbonyldiimidazole in portions 128.7g, during which the temperature was controlled at -20°C to -25°C. After the addition was completed, the reaction was maintained at -20°C to -25°C for 45 minutes; 116.0g of 2,6-dichloro-3-amino-4-methylpyridine was dissolved In 750mL of dry toluene, then drop into the active amide solution in the previous step, maintain -15°C to -20°C during the period, and react at -15°C to -20°C for 2 hours after the addition; after TLC checks that the reaction is complete, add 500mL Stirring with water, the solid precipitated out and was filtered. The solid was washed with 100mL water and 100mL toluene respectively. After vacuum drying, 169.0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com