Preparation method of 4-(4-dimethylaminostyryl)methylpyridyl p-toluenesulfonate
A technology of dimethylaminostyrene and p-toluenesulfonate, which is used in the preparation of 4-(4-dimethylaminostyryl)methylpyridine p-toluenesulfonate, and the preparation of high-quality organic nonlinear optics In the field of crystals, it can solve the problems of complicated synthesis steps, limited crystal growth, and long reaction time, and achieve the effects of simple preparation method, short reaction time, and simple reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
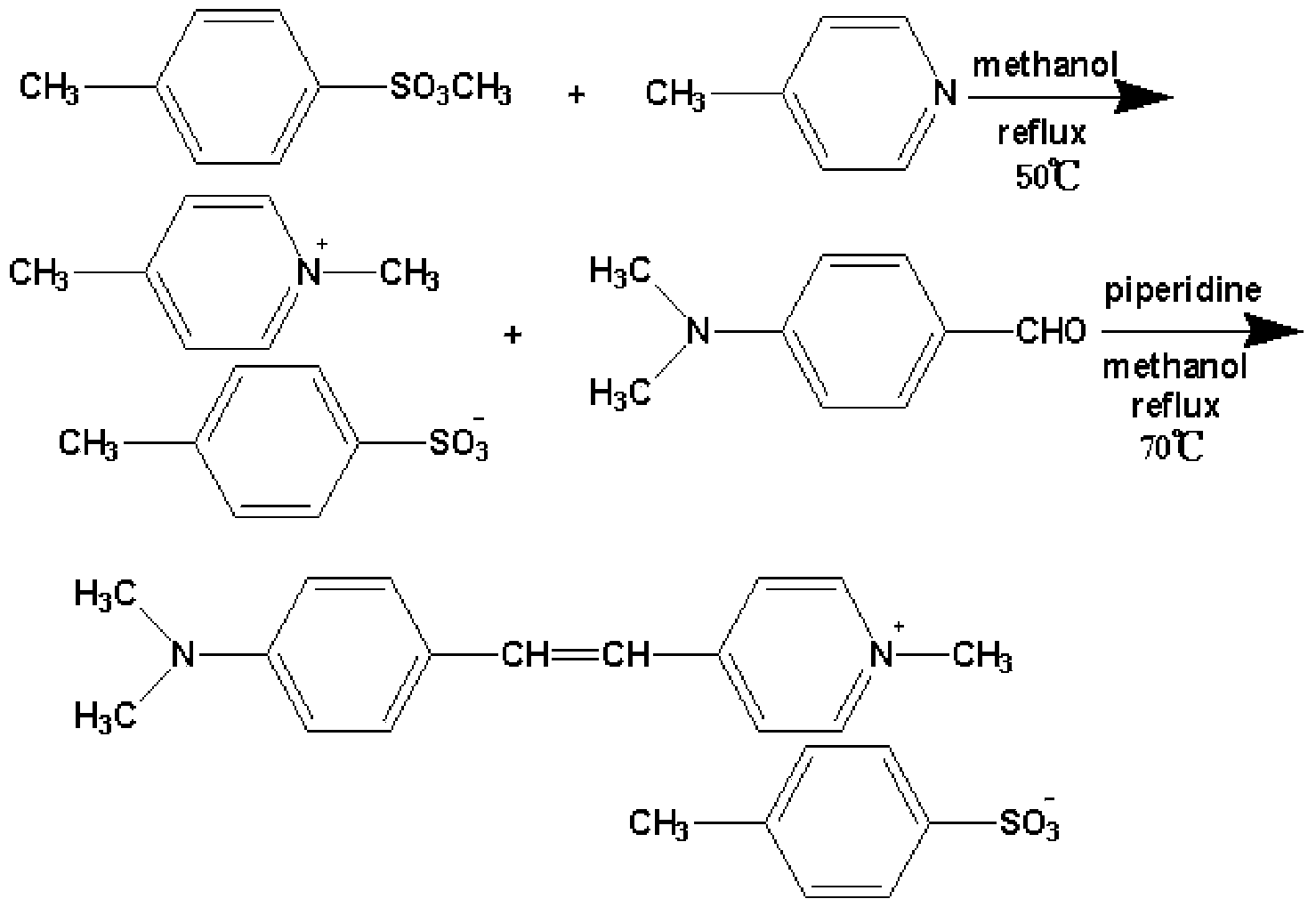
[0018] The specific steps for preparing 4-(4-dimethylaminostyryl)picoline p-toluenesulfonate in this example include three steps: ion exchange reaction, condensation reaction, and filtration purification:
[0019] (1) Ion exchange reaction: at room temperature, mix 0.05mol 4-picoline (purity 98%) and 0.05mol methyl p-toluenesulfonate and place in 50-60ml anhydrous methanol (purity not less than 99.5 %), then mix the two solutions and put them in a 250ml three-hole flask, heat and stir slowly to 50°C, ion exchange reaction for 12-18h, and condense and reflux at the same time, the solution does not change color during the ion exchange reaction, and the reaction Obtain 4-methyl-N-methylpyridine p-toluenesulfonate;
[0020] (2) Condensation reaction: Weigh 0.05mol of p-dimethylaminobenzaldehyde (analytically pure) and dissolve it in 50-60ml of anhydrous methanol (purity not less than 99.5%), heat and stir until it is completely dissolved, Use a glass rod to drain and put it into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com