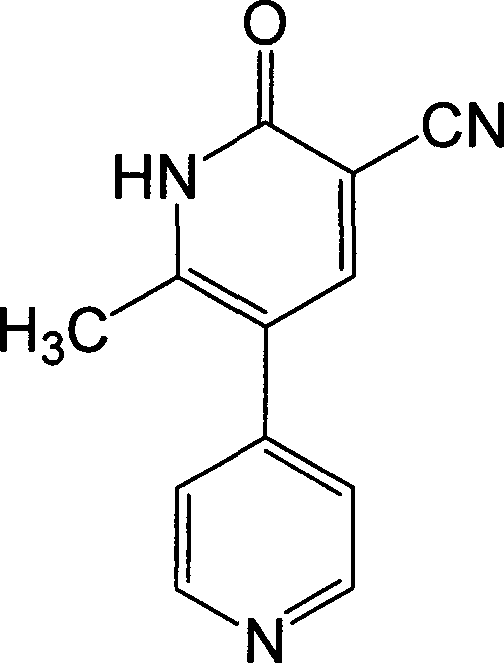
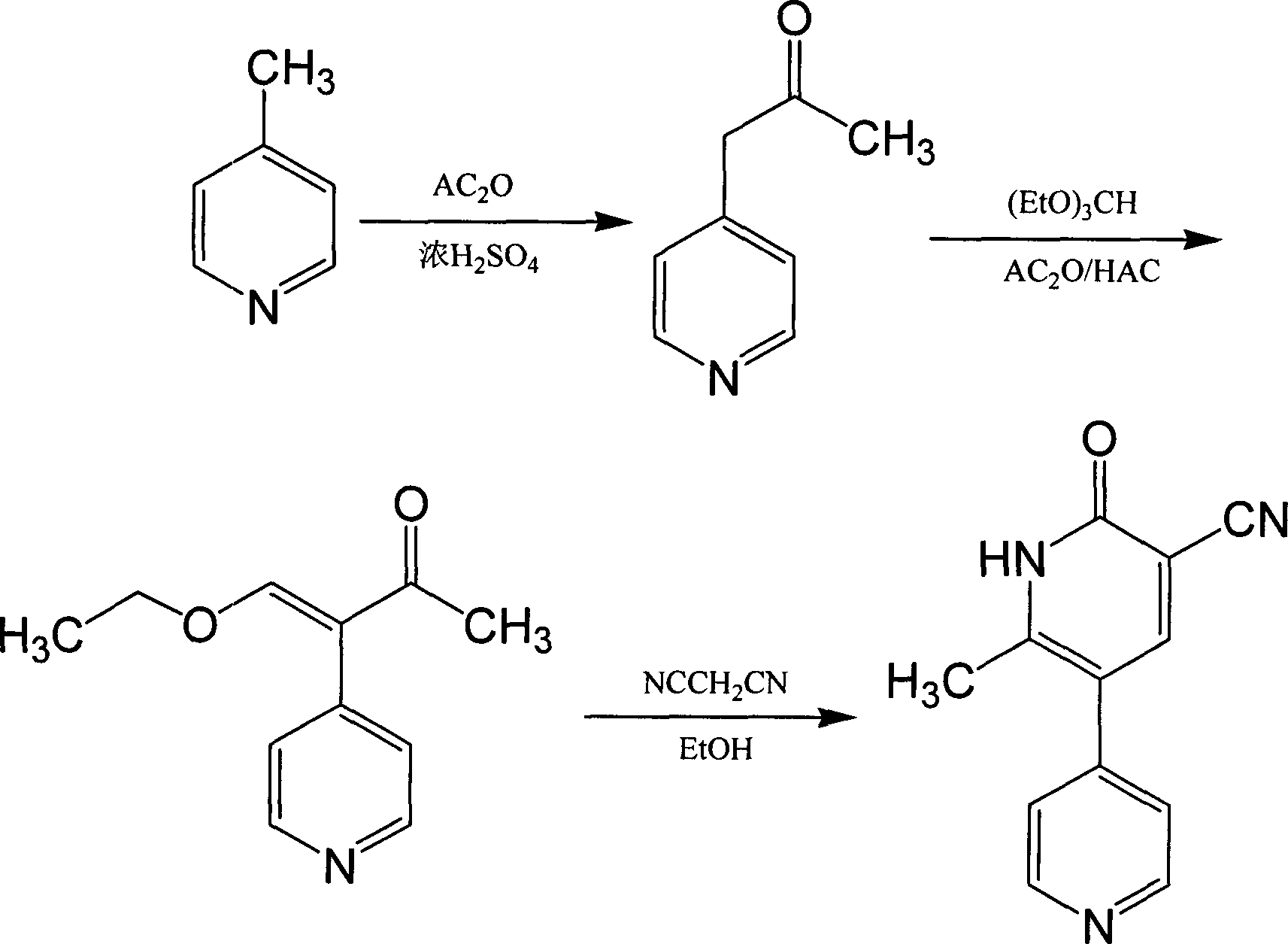
Synthesis method of milirinone
A synthesis method and condensation reaction technology, applied in organic chemistry and other directions, can solve the problems of expensive raw materials, complex synthesis routes, and high equipment requirements, and achieve the effects of simplifying operations, simplifying technological processes, and reducing production environment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Take 192g of 4-picoline, 662g of acetic anhydride, and 1.5ml of concentrated sulfuric acid in a three-necked flask, place a thermometer, stir, heat up to 75°C for 4 hours, cool down and add 850ml of absolute ethanol, reflux for 2 hours, and depressurize concentrate. Then add 163g of triethyl orthoformate, 184g of acetic anhydride, and 150ml of glacial acetic acid in sequence, keep the temperature in the bottle at 50°C, react for 2.5 hours, concentrate under reduced pressure, then add 1000ml of absolute ethanol, 54.2g of malononitrile, and heat to reflux. Dissolve with alkaline solution at room temperature, decolorize activated carbon, filter, and soak twice with purified water, adjust pH to 6.5-7.0 with glacial acetic acid, filter and dry, and dry to obtain 288 g of crude product, then recrystallize with DMF, collect milrinone Refined product 254g, the yield is 58.3%.
example 2
[0018] Take 145g of 4-picoline, 500g of acetic anhydride, and 1.2ml of concentrated sulfuric acid in a three-necked flask, place a thermometer, stir, heat up to 75°C for 4 hours, cool down and add 650ml of absolute ethanol, reflux for 2 hours, and depressurize concentrate. Then add 115g of triethyl orthoformate, 140g of acetic anhydride, and 115ml of glacial acetic acid in sequence, keep the temperature in the bottle at 50°C, react for 2.5 hours, concentrate under reduced pressure, then add 800ml of absolute ethanol, 40.9g of malononitrile, and heat to reflux. Dissolve with alkaline solution at room temperature, decolorize with activated carbon, filter, and soak twice with purified water, adjust pH to 6.5-7.0 with glacial acetic acid, dry by suction, dry to obtain 216g of crude product, recrystallize with DMF, and collect milrinone Refined product 188g, the yield is 57.2%.
example 3
[0020] Take 950g of 4-picoline, 3350g of acetic anhydride, and 8ml of concentrated sulfuric acid in a reaction flask, place a thermometer, stir, heat up to 75°C for 4 hours, cool down and add 4250ml of absolute ethanol, reflux for 2 hours, and concentrate under reduced pressure. Then add 815g of triethyl orthoformate, 920g of acetic anhydride, and 750ml of glacial acetic acid in sequence, keep the temperature in the bottle at 50°C, react for 2.5 hours, concentrate under reduced pressure, then add 5000ml of absolute ethanol, 270g of malononitrile, and heat to reflux. Dissolve with alkaline solution at room temperature, decolorize with activated carbon, filter, and soak twice with purified water, adjust pH to 6.5-7.0 with glacial acetic acid, filter to dryness, and dry to obtain 1418g of crude product, then recrystallize with DMF, collect milrinone Refined product 1241g, the yield is 57.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com