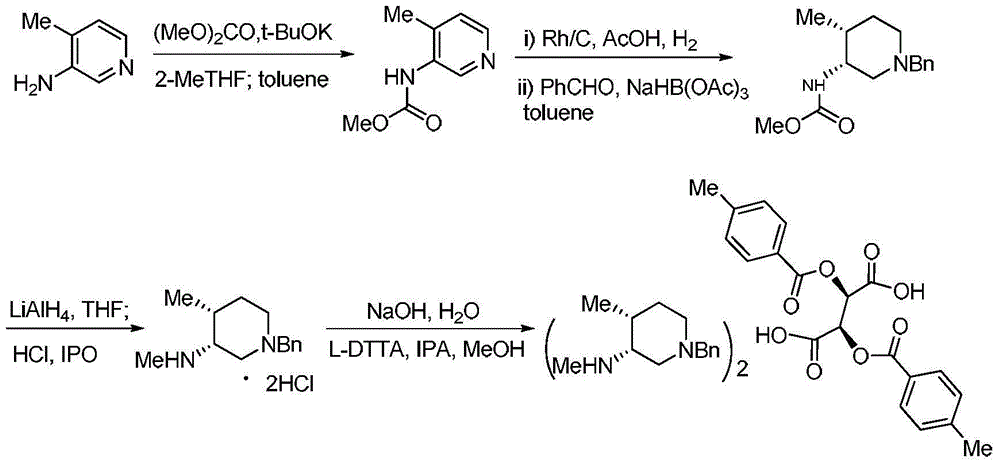
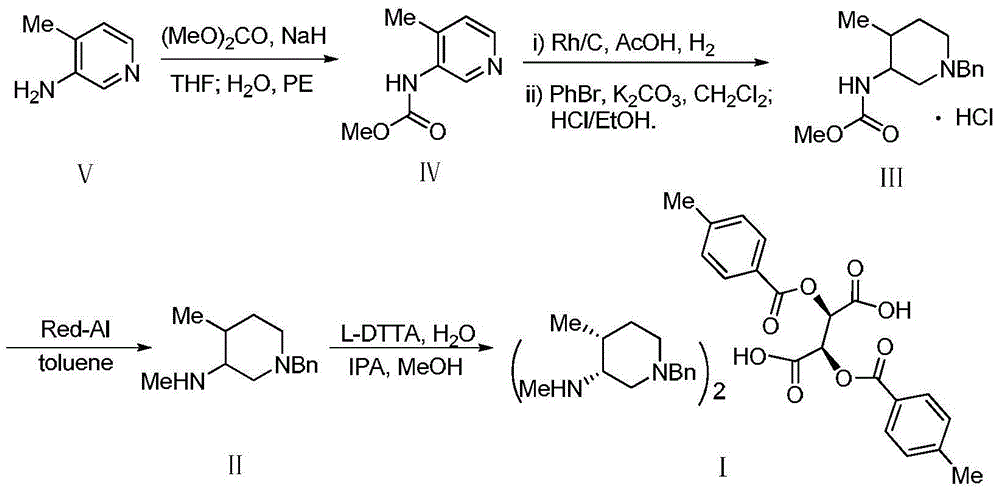
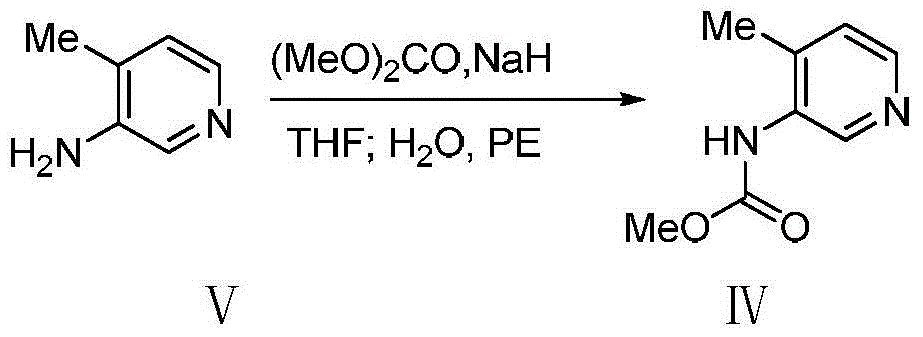Bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate synthesis method
A technology of toluyl tartrate and dimethylpiperidine, which is applied in the field of chemical pharmacy, can solve the problems of unsatisfactory cost control and environmental protection, inconvenient transportation and storage, and increased catalyst cost, and achieve effective Conducive to environmental protection, satisfactory product quality and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Sodium hydride (8.0g, 200mmol) was suspended in anhydrous tetrahydrofuran, and slowly added dropwise to a solution of compound V (21.6g, 200mmol) in anhydrous tetrahydrofuran (150mL) at -5 ~ 5°C, and the stirring was continued for 60min, then Slowly add dimethyl carbonate (19.80g, 220mmol) dropwise. After the dropwise addition, stir at 40°C for 7h. TLC shows that the reaction is complete. Slowly add 30mL of distilled water, stir for 30min, and evaporate the organic solvent under reduced pressure. Add 20mL of petroleum ether, stir vigorously under ice bath for 30min, filter, wash the filter cake with petroleum ether (30mL×3), and dry to obtain light brown product IV (31.0g), yield: 93.4%, purity (HPLC): 98.7%, 1 H NMR (400MHz, CDCl 3 )δ: 2.27(s, 3H), 3.8(s, 3H), 6.39(s, 1H), 7.10(d, J=5.2Hz, 1H), 8.27(d, J=4.8Hz, 1H), 8.86( s,1H).ESI-HRMS: Calcd for C 8 h 10 N 2 o 2 (M+H) + 167.0815, found 167.0818.
Embodiment 2
[0022]
[0023] Sodium hydride (12.0 g, 300 mmol) was suspended in anhydrous tetrahydrofuran, and slowly added dropwise to a solution of compound V (21.6 g, 200 mmol) in anhydrous tetrahydrofuran (150 mL) at -5 to 5 ° C, and stirring was continued for 60 min, then Slowly add dimethyl carbonate (19.80g, 220mmol) dropwise, stir at room temperature for 10h after the dropwise addition, TLC shows that the reaction is complete, slowly add 50mL of distilled water, stir for 30min, evaporate the organic solvent under reduced pressure, and add 20mL petroleum ether, stirred vigorously for 30min under ice bath, filtered, the filter cake was washed with petroleum ether (30mL×3), dried to obtain light brown product IV (31.2g), yield: 94.1%, purity (HPLC): 97.9% .
[0024] Reference Example 1
[0025]
[0026] The acetic acid solution (200mL) of compound IV (33.2g, 200mmol) was added to the acetic acid (100mL) solution of 5%Rh / C (4.2g), stirred for 15min, and in a hydrogen atmospher...
Embodiment 3
[0031]
[0032] Dissolve compound III (3.0g, 10mmol) in toluene (30mL), slowly add red aluminum toluene solution (8.7g) dropwise under ice-water bath, after the addition is complete, react at room temperature for 2h, then cool the reaction solution below 10°C , slowly added distilled water (30mL) dropwise under vigorous stirring, stirred for 30min, filtered, the filter cake was washed with toluene (20mL×3), the filtrate was extracted with toluene, the organic phases were combined, dried, and the solvent was removed by rotary evaporation, the residue (cis structure Purity (GC): 93.2%) was directly used in the next reaction without purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com