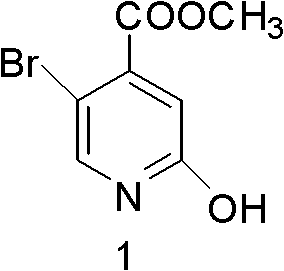
Synthesis method of 5-bromo-2-methyl 4-hydroxypyridinecarboxylate
A technology of hydroxyisonicotinic acid and methyl ester, applied in the field of chemistry, can solve the problems of low total yield, no literature report on the synthesis method, difficulty in large-scale industrial production, etc., and achieves simple and easy operation of reaction steps and safe and reliable reaction process. , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
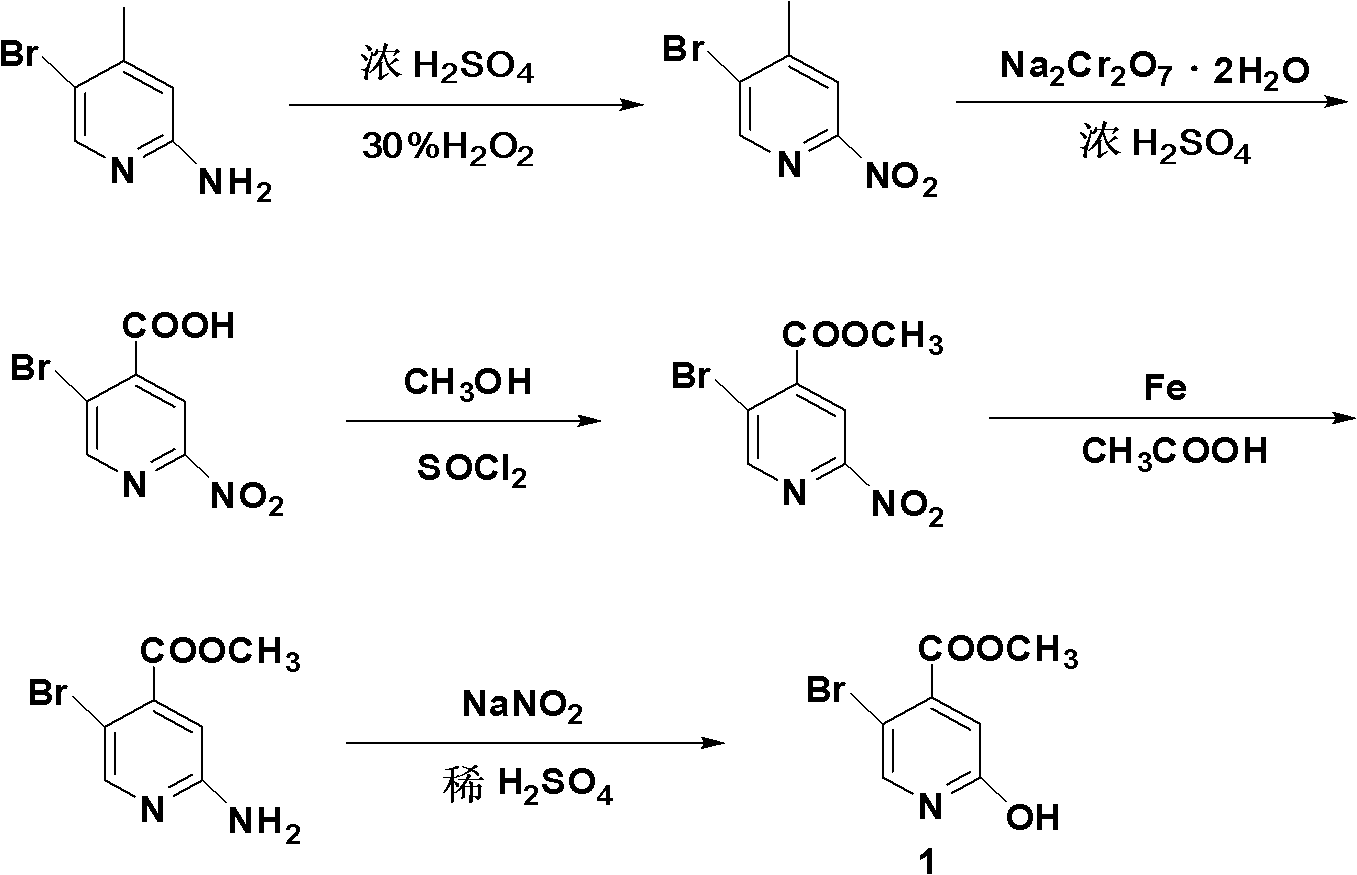
[0037] (1) Synthesis of 5-bromo-4-methyl-2-nitropyridine
[0038]Slowly add 1500ml of concentrated sulfuric acid (98.3% in mass fraction) dropwise into a 5L Erlenmeyer flask containing 750ml of 30% hydrogen peroxide. During the addition, the temperature is controlled to be less than or equal to 20°C. After the addition, cool to Standby at 5°C; add 0.535mol 2-amino-5-bromo-4-picoline to 2550ml of concentrated sulfuric acid, and cool down to zero; slowly add the stock solution prepared in the previous step to the mixture, and control the temperature at 5°C ℃15℃, stirring at 5℃15℃ after dripping for 30 minutes, then reacting at room temperature for 3 hours; after the reaction, pour the reaction solution into the ice-water mixture, adjust the pH to 7-8 with NaOH solution, and precipitate a large amount of light yellow The solid was filtered with suction, and the filter cake was washed with a small amount of water; dried at 45°C to obtain 104.6 g of 5-bromo-4-methyl-2-nitropyridine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com