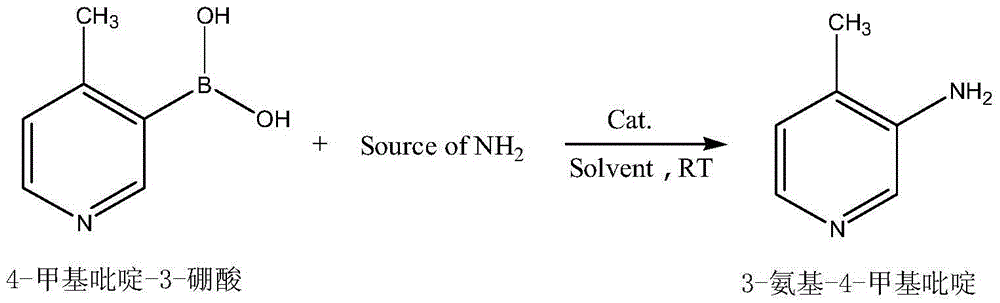
Preparation method of 3-amino-4-methylpyridine
A picoline and amino technology, applied in the field of organic chemical synthesis, can solve the problems of difficult preparation, harsh reaction conditions, and low product yield, and achieve the effects of simple reaction process, mild reaction conditions, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a flask equipped with a mechanical stirring device, add 27.4g (0.2mol) of 4-picoline-3-boronic acid, 50ml of methanol, 128g (1mol) of ammonia (mass concentration 28%), 2.9g (0.02mol) of oxynitride Copper, start stirring, and the reaction is completed in 2 hours at room temperature (TLC monitors the reaction process). After suction filtration, the filtrate was concentrated under reduced pressure, and the obtained solid was recrystallized with ethyl acetate to obtain 20.5 g of 3-amino-4-methylpyridine, with a yield of 95%.
[0021] Example 1
[0022] In a flask equipped with a mechanical stirring device, add 27.4g (0.2mol) of 4-picoline-3-boronic acid, 50ml of methanol, 128g (1mol) of ammonia (mass concentration 28%), 2.3g (0.01mol) of silver oxide , Stirring was started, and the reaction was completed in 1 h at room temperature (TLC monitored the reaction process). After suction filtration, the filtrate was concentrated under reduced pressure, and the obtained solid...
Embodiment 3
[0024] In a flask equipped with a mechanical stirring device, add 27.4g (0.2mol) 4-picoline-3-boronic acid, 50ml acetonitrile, 75ml water, 39.6g (0.3mol) ammonium sulfate, 1.6g (0.02mol) copper oxide , Stirring was started, and the reaction was completed in 4 h at room temperature (TLC monitored the reaction process). After suction filtration, the filtrate was concentrated under reduced pressure, and the obtained solid was recrystallized with ethyl acetate to obtain 18.3 g of 3-amino-4-methylpyridine, with a yield of 85%.
Embodiment 4
[0026] In a flask equipped with a mechanical stirring device, add 27.4g (0.2mol) 4-picoline-3-boronic acid, 100ml ethanol, 100ml water, 21.4g (0.4mol) ammonium chloride, 1.6g (0.04mol) oxidation Zinc, start stirring, and the reaction is completed in 6 hours at room temperature (TLC monitors the reaction process). After suction filtration, the filtrate was concentrated under reduced pressure, and the obtained solid was recrystallized with ethyl acetate to obtain 18.1 g of 3-amino-4-methylpyridine, with a yield of 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com