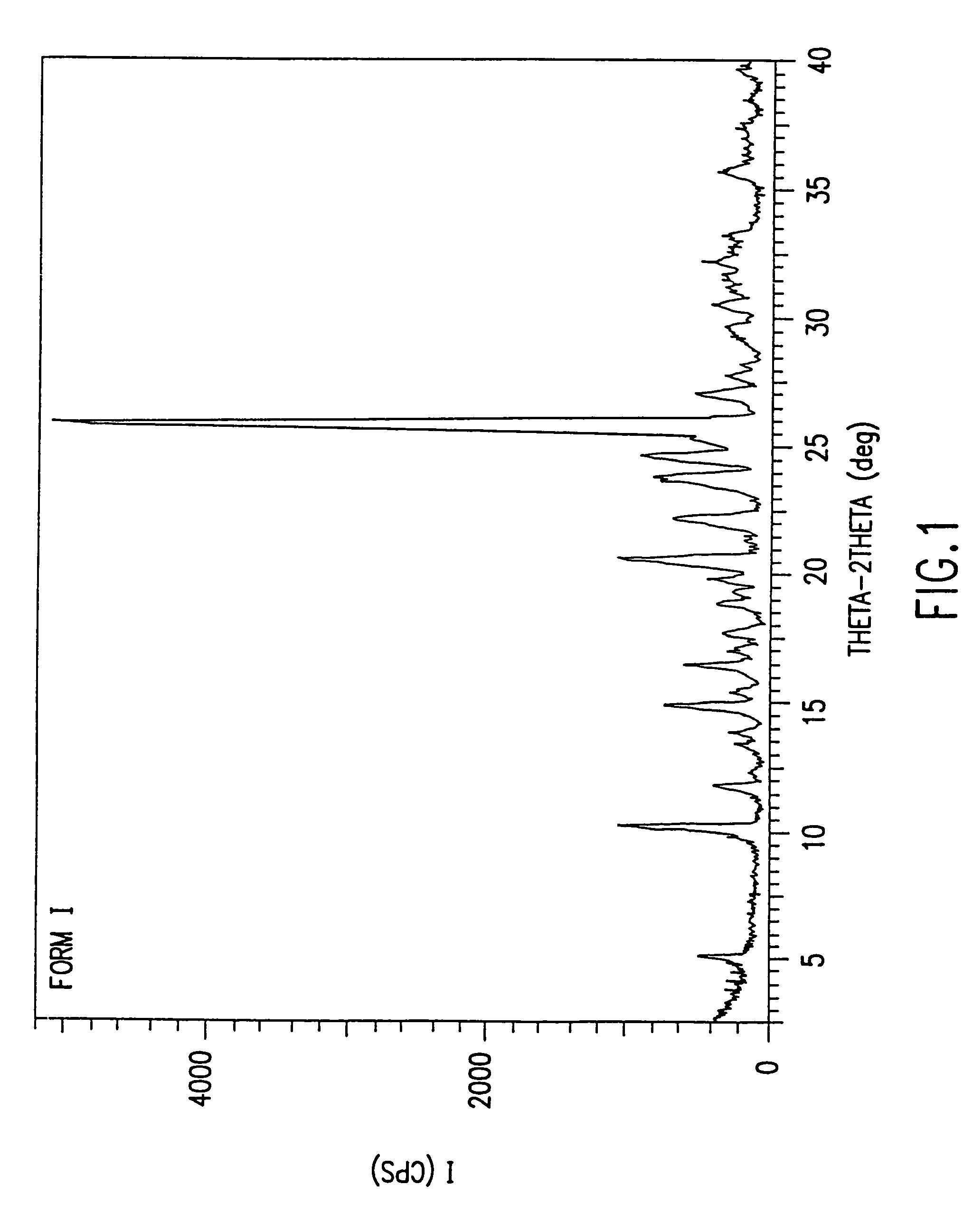
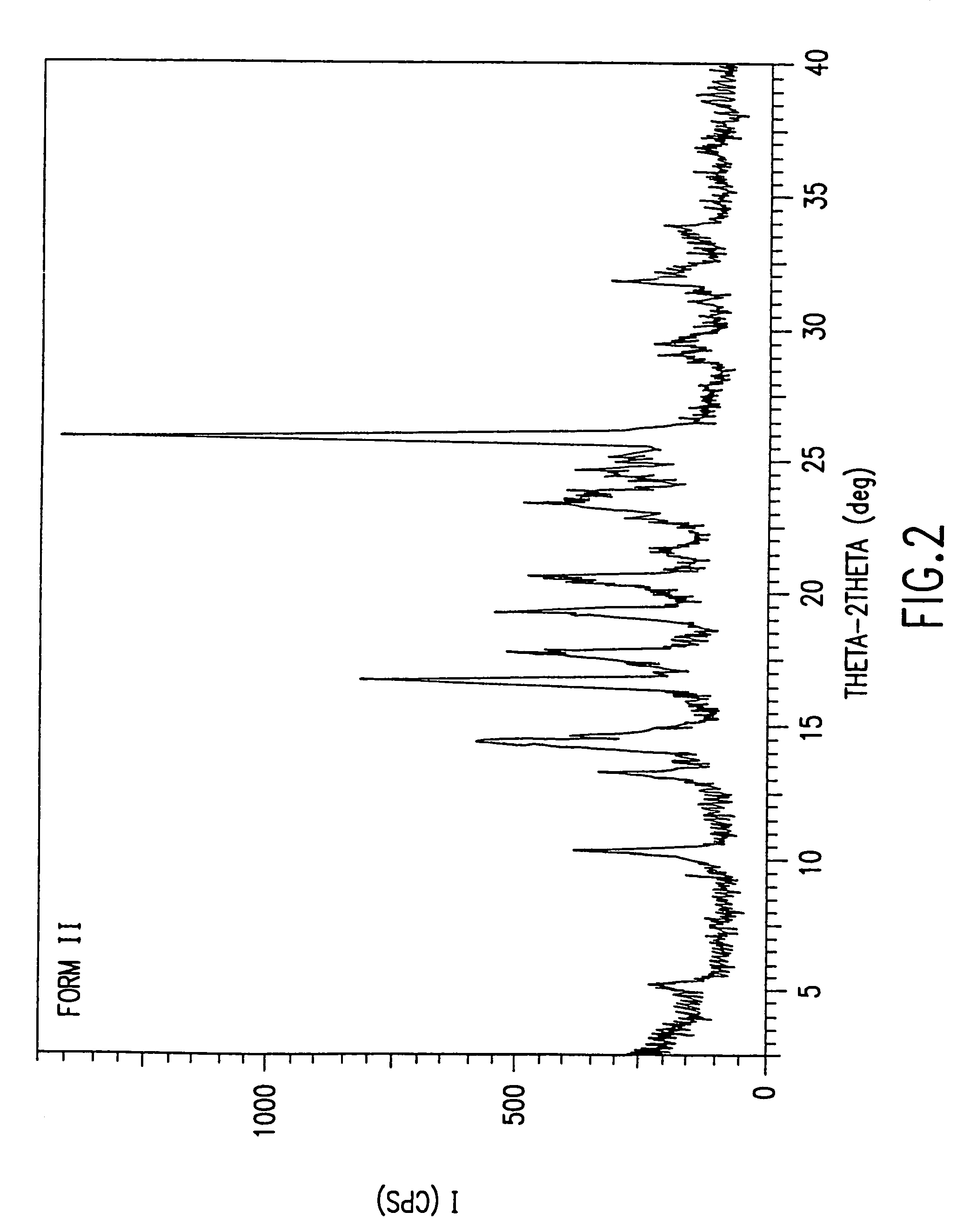
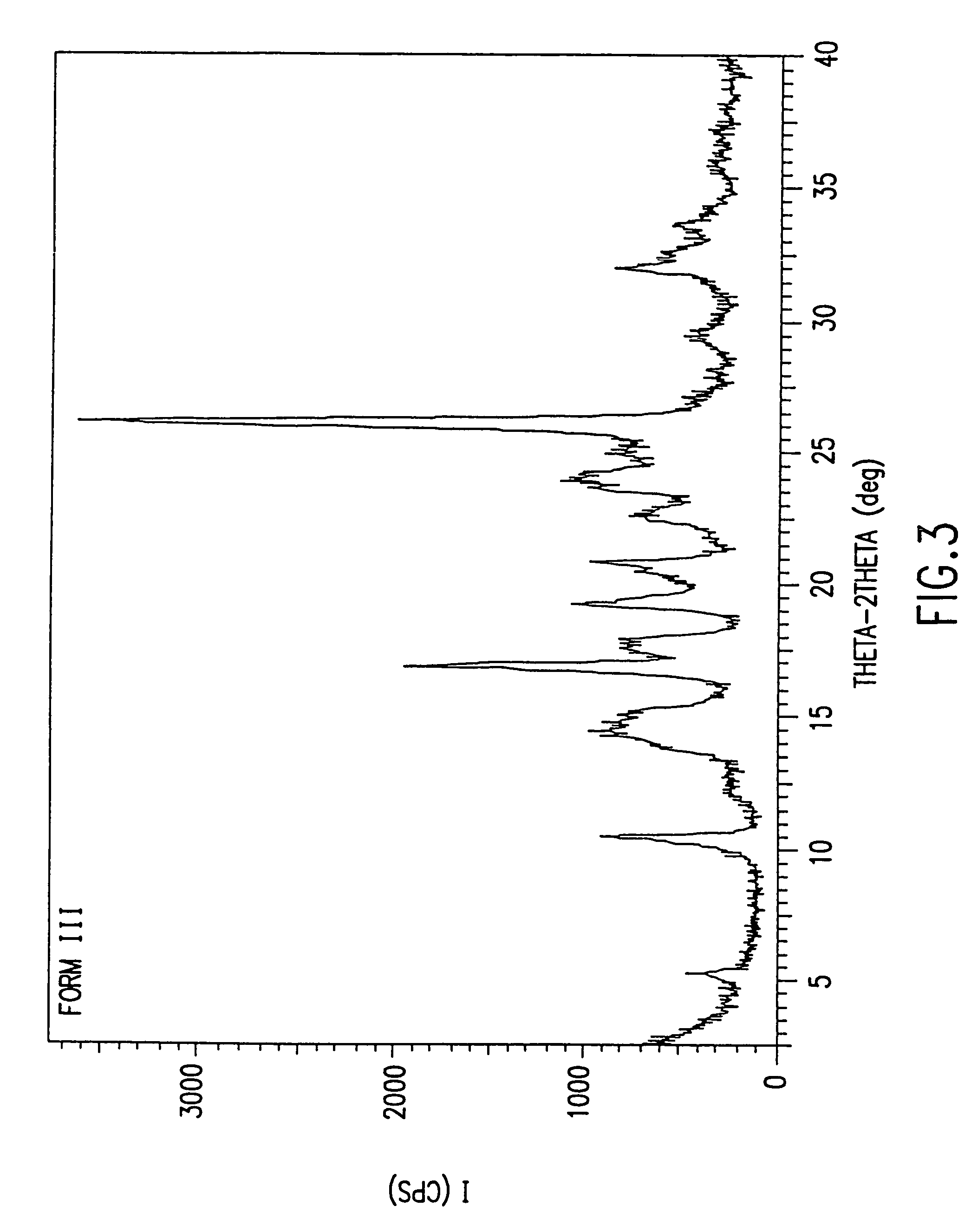
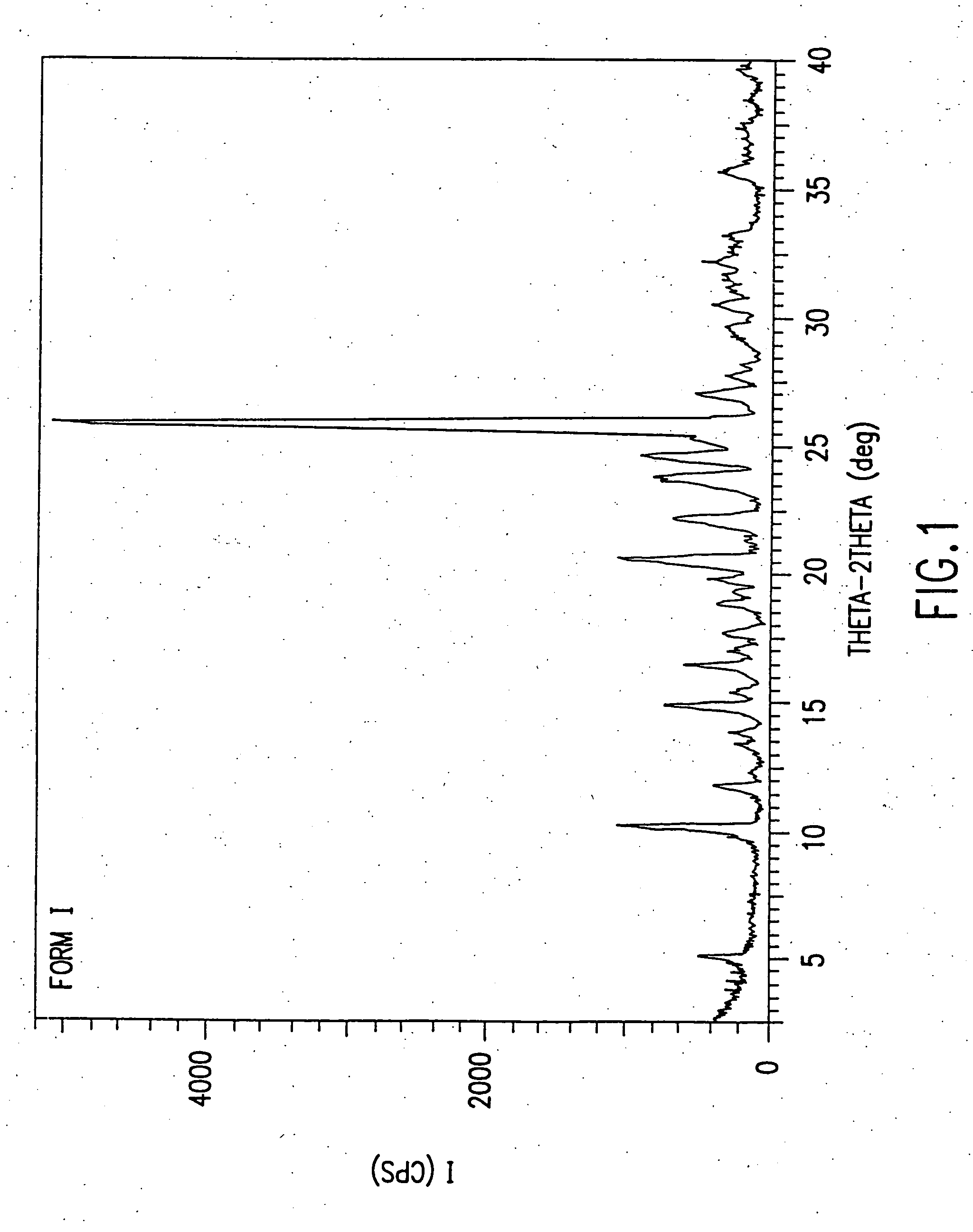
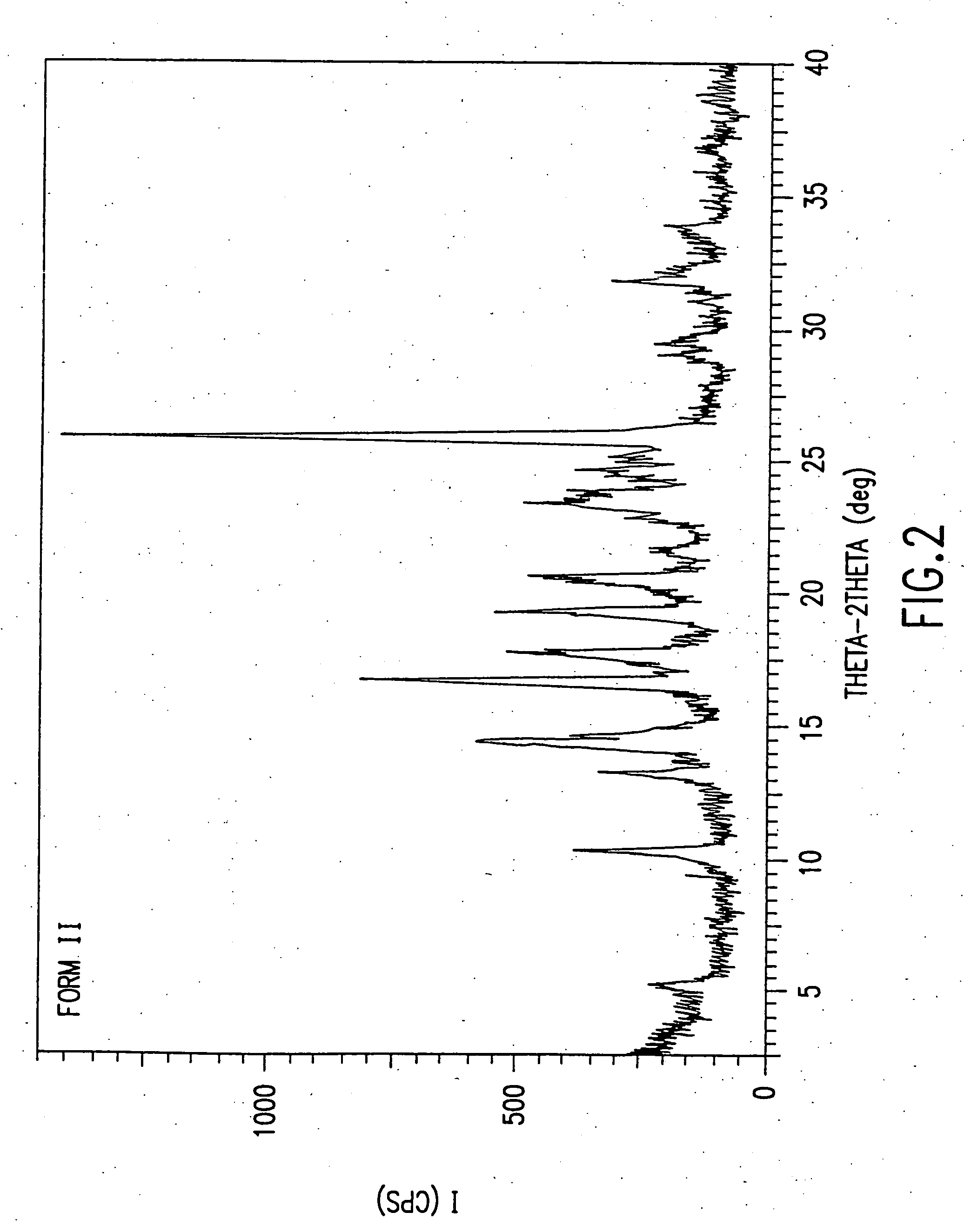
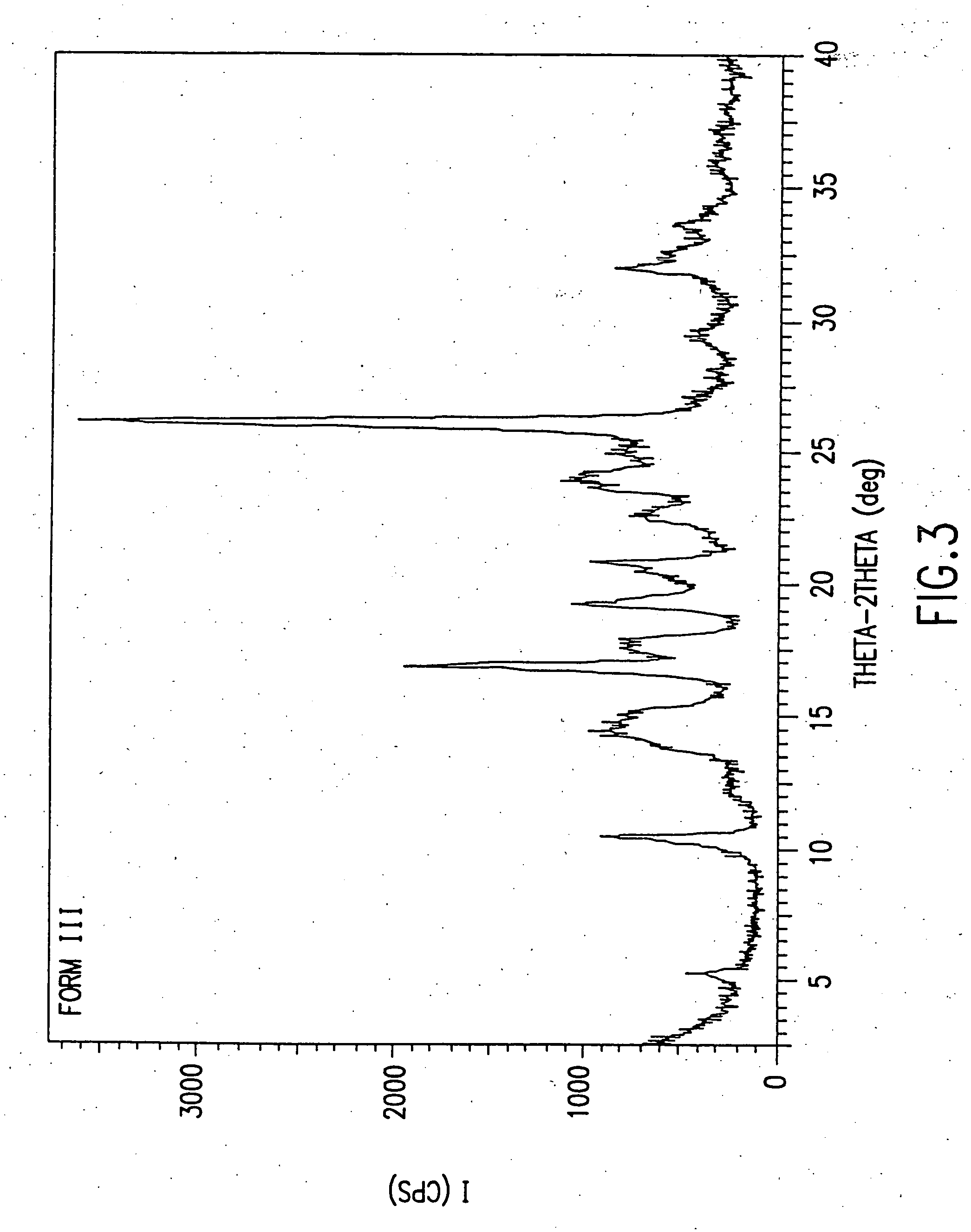
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
274 results about "Desmethyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Desmethyl is a term used in organic chemistry to refer to a methyl group that has been removed. Examples include desmethyltramadol and desmethylsertraline.
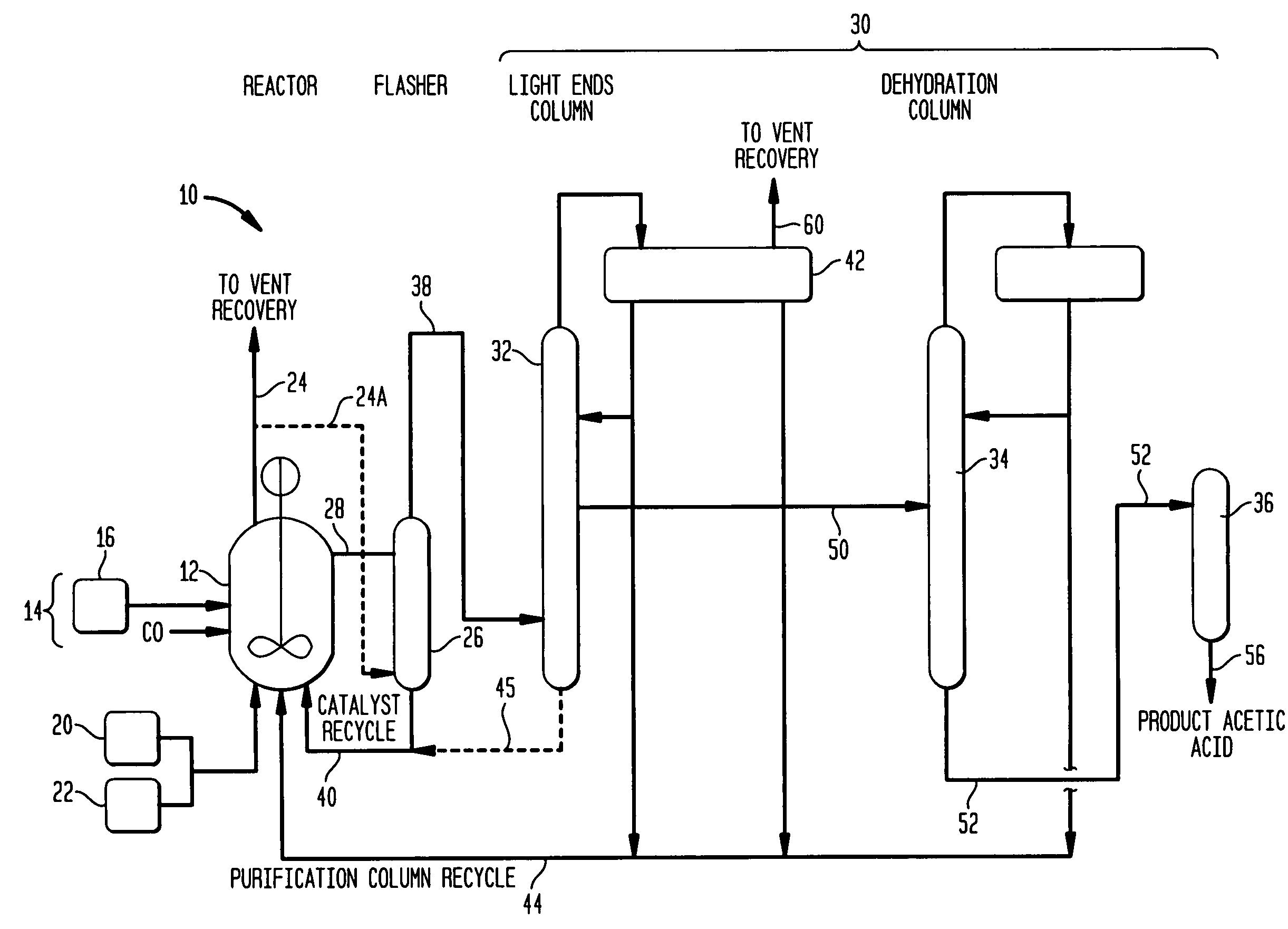
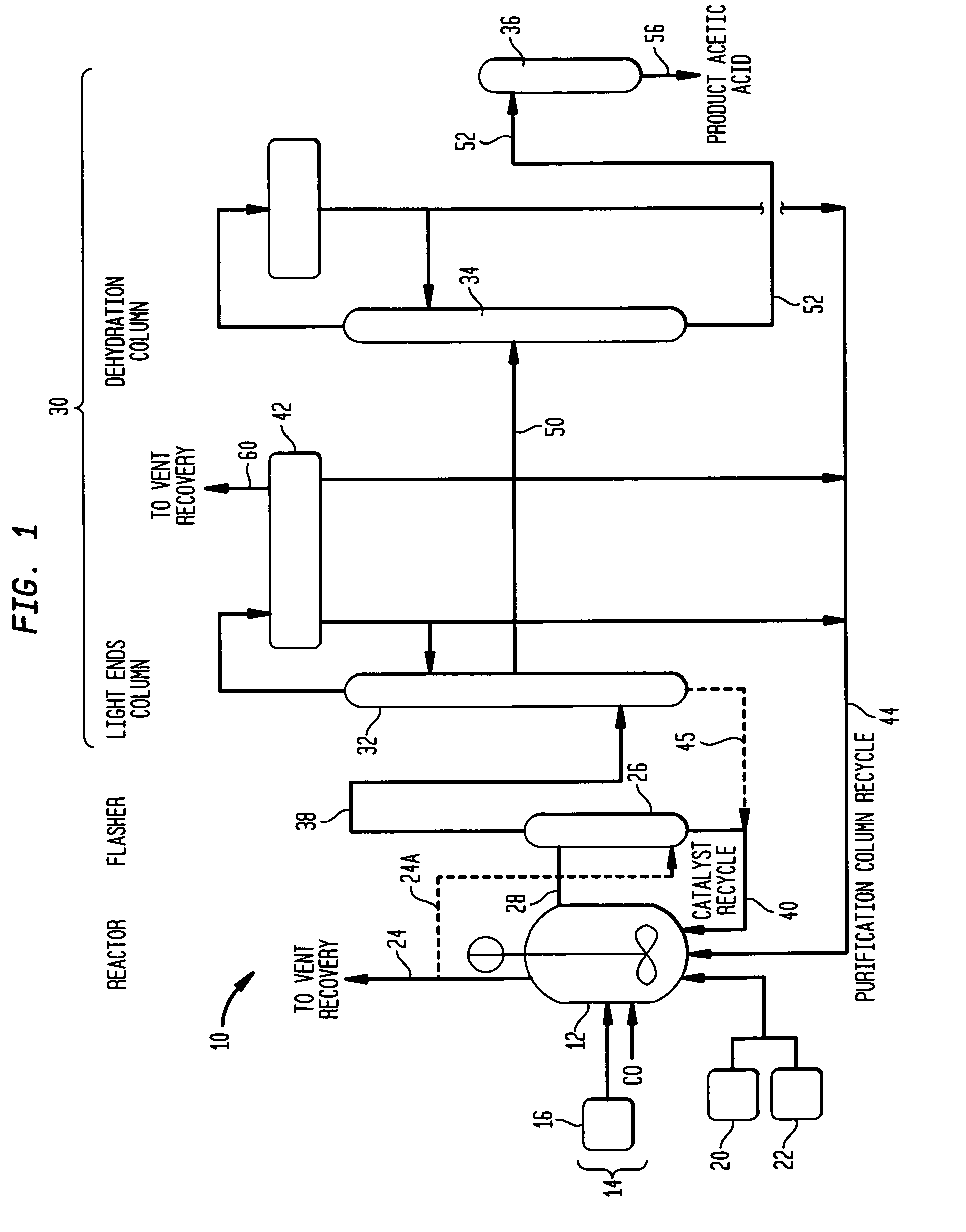
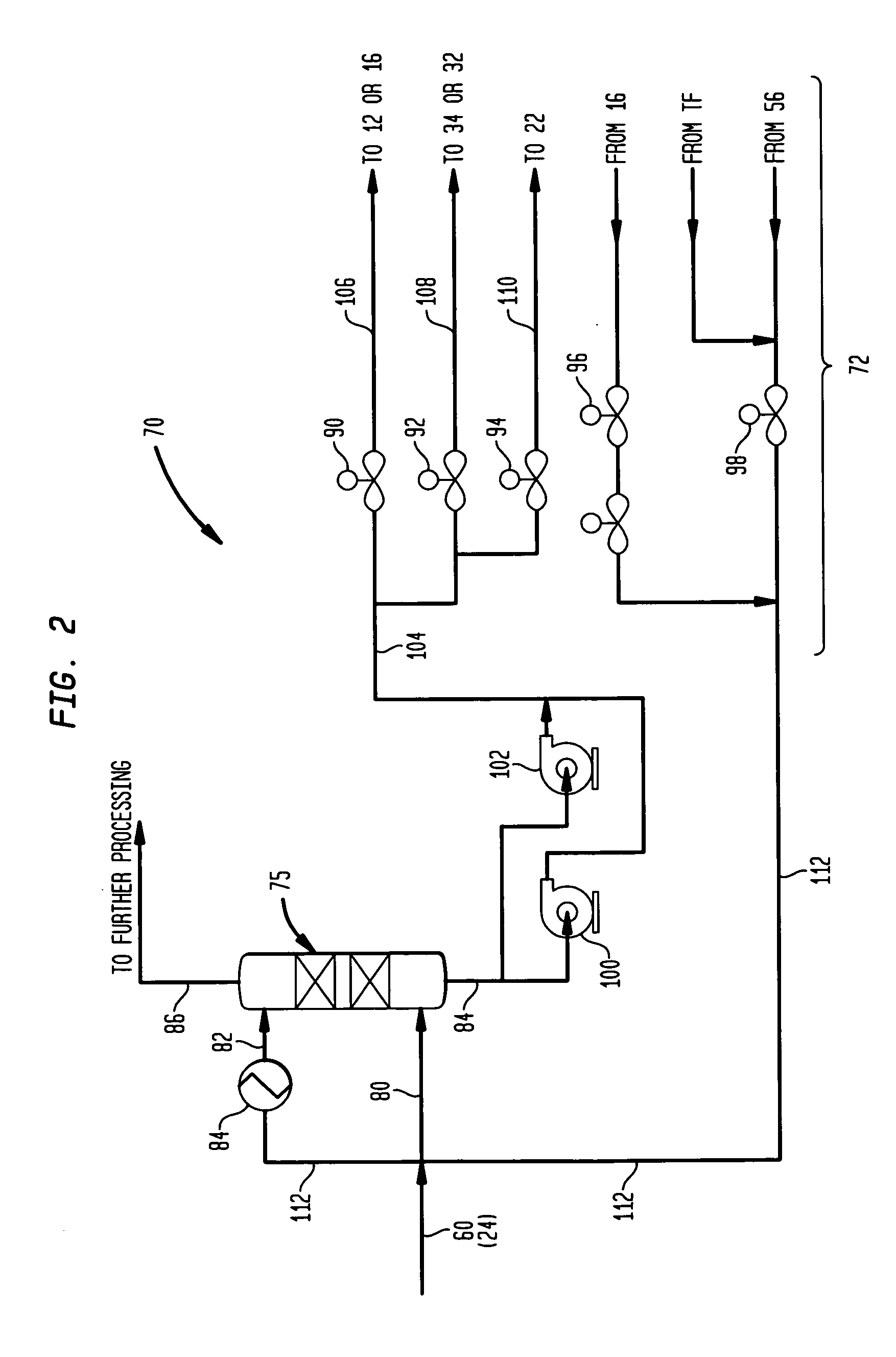
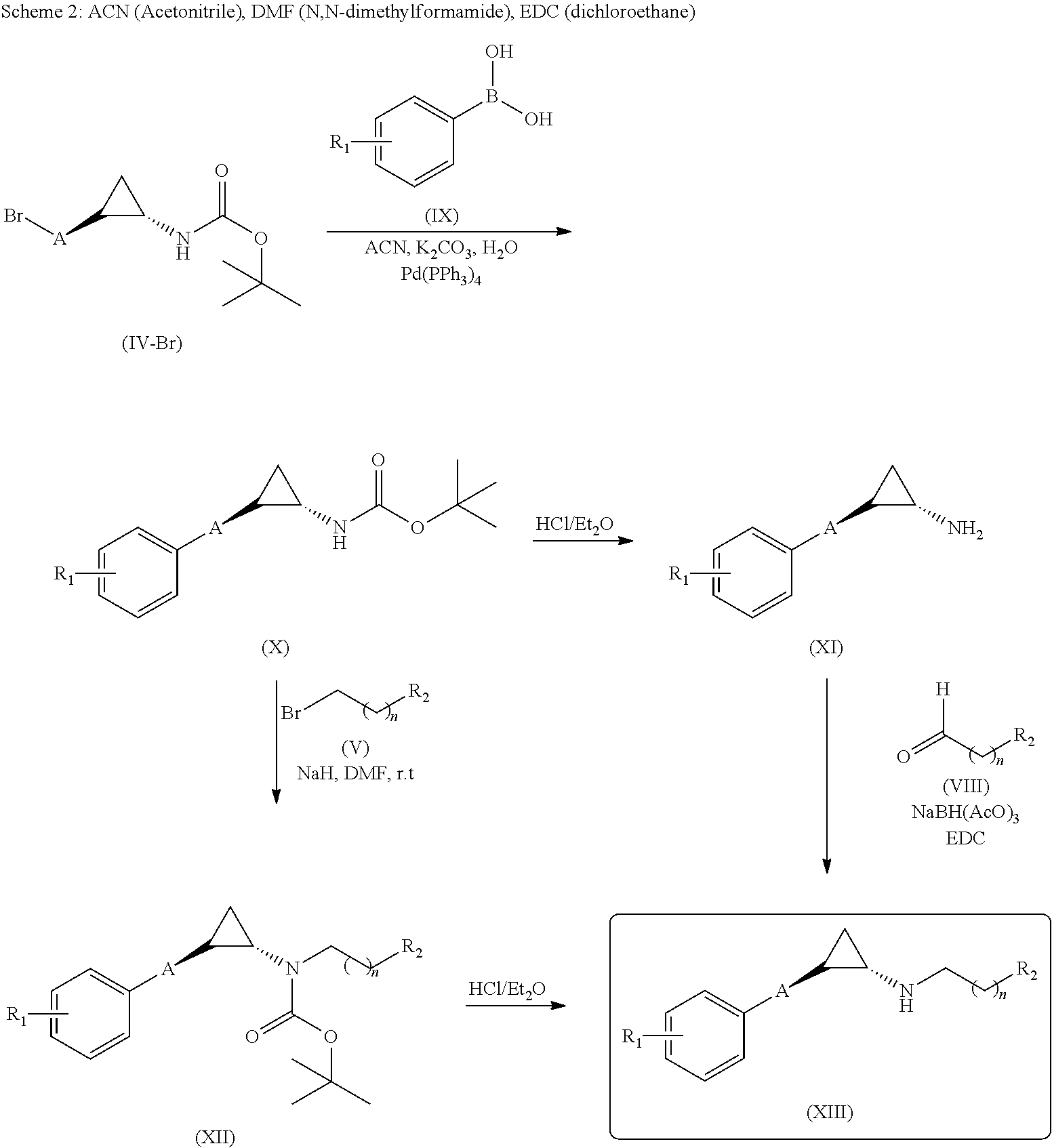
Methanol carbonylation system having absorber with multiple solvent options
A methanol carbonylation system 10 includes an absorber tower 75 adapted for receiving a vent gas stream and removing methyl iodide therefrom with a scrubber solvent, the absorber tower being coupled to first and second scrubber solvent sources 16, 56 which are capable of supplying different first and second scrubber solvents. A switching system including valves 90, 92, 94, 96, 98 alternatively provides first or second scrubber solvents to the absorber tower and returns the used solvent and sorbed material to the carbonylation system to accommodate different operating modes.
Owner:CELANESE INT CORP
Inhibitors of histone demethylases
Compounds of the formIn which Q is selected from —CH═NR12, —W, —CH2NHR13, —CH═O and —CH(OR17)2 capable of modulating the activity of histone demethylases (HDMEs), which are useful for prevention and / or treatment of diseases in which genomic dysregulation is involved in the pathogenesis, such as e.g. cancer and formulations and methods of use of such compounds.
Owner:GILEAD SCI INC
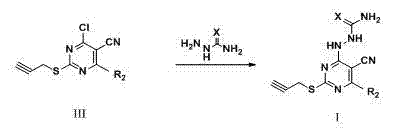
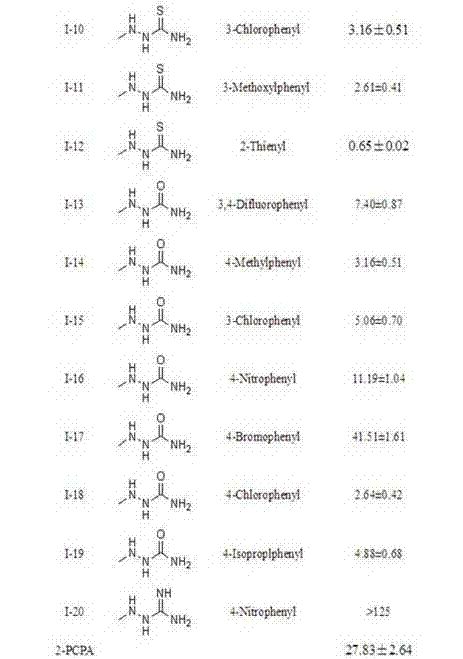
Pyrimidine derivatives containing semicarbazide and terminal alkyne structural units, and preparation methods and applications of pyrimidine derivatives
ActiveCN104119280AHigh activityMild reaction conditionsOrganic active ingredientsOrganic chemistryAlkynePharmaceutical Substances
The invention belongs to the field of medicinal chemistry, and discloses pyrimidine compounds containing semicarbazide and terminal alkyne structural units, and preparation methods and applications of the pyrimidine compounds in preparation of antitumor drugs by taking lysine specific demethylase 1 (hereafter referred to as LSD1) as a target. A pyrimidine active fragment is built by adopting a three-component one-pot method, and then the target compounds are prepared by substitution, chlorination and ammonification reaction. The general formulas of the compounds are as shown in the formula I in the specification. An in vitro anti-tumor activity experiment and an LSD1 inhibition activity experiment prove that the compounds have obvious inhibiting and killing action on a plurality of tumor cells by inhibiting the activity of the LSD1, can be used as lead compounds for further development, and are applied to preparation of the antitumor drugs.
Owner:ZHENGZHOU UNIV
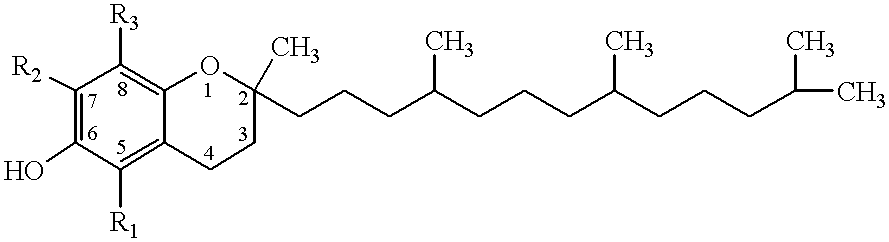
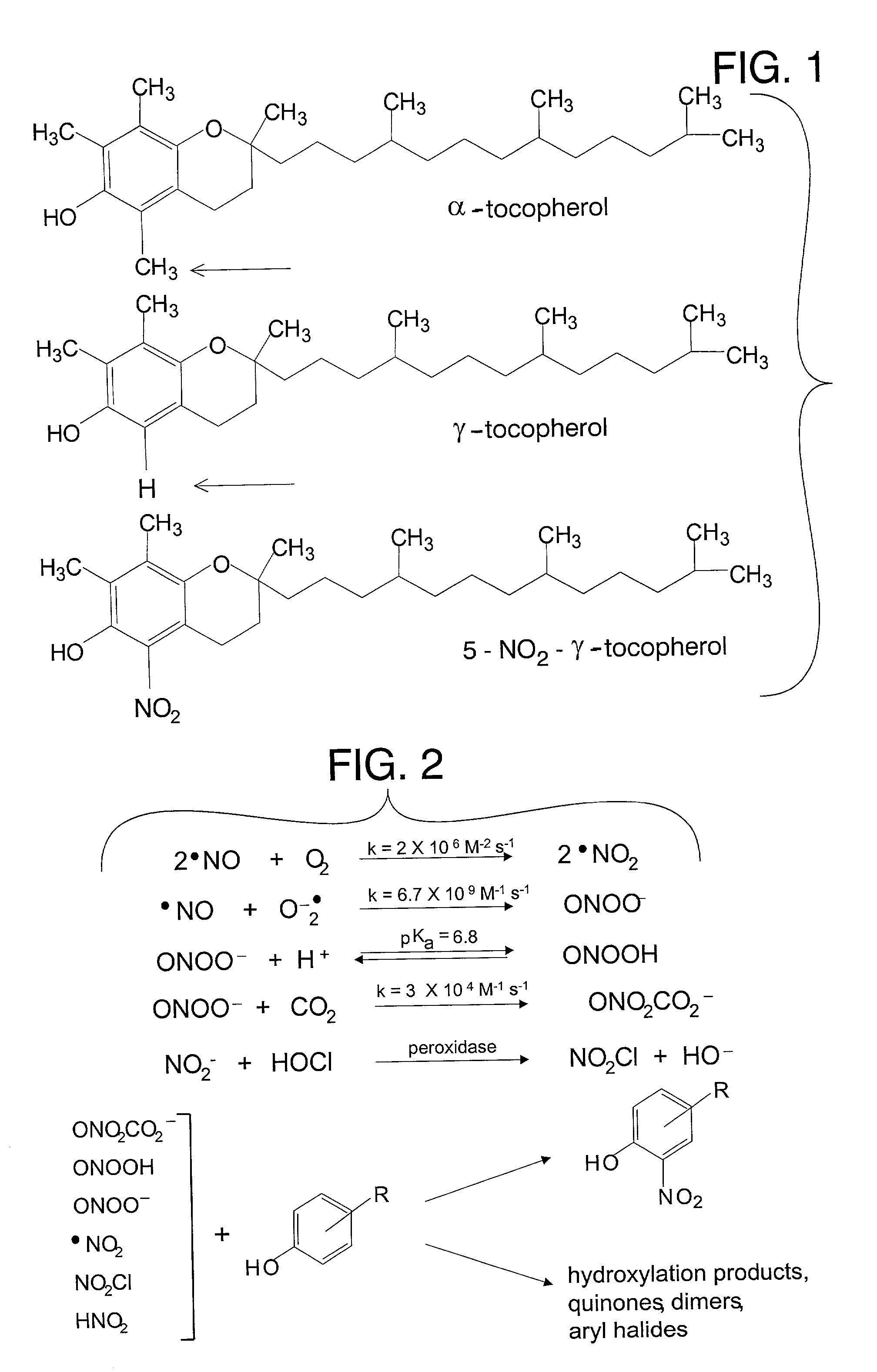
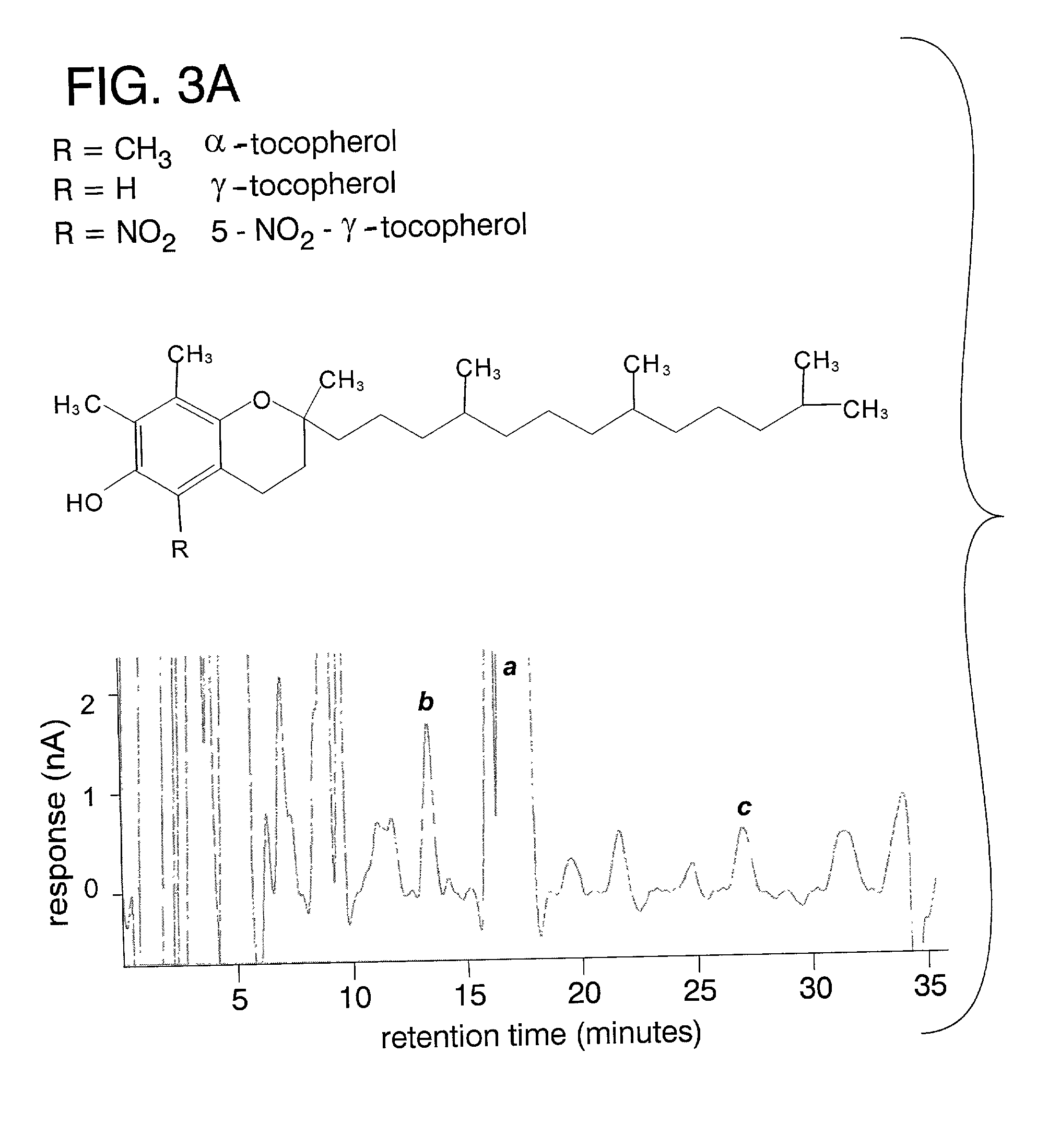
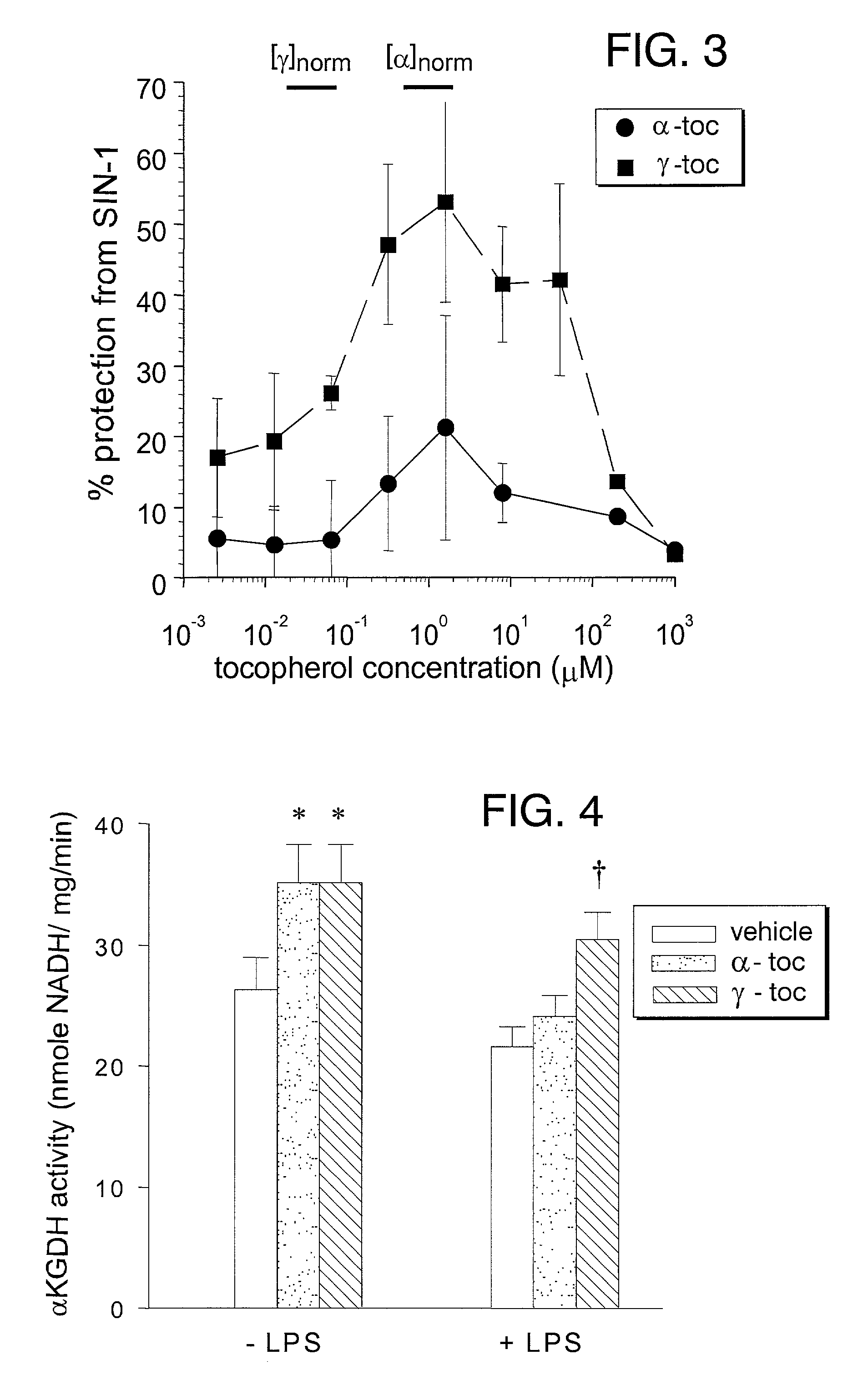
Desmethyl tocopherols for preventing or slowing degenerative neurological diseases
InactiveUS20020006954A1BiocideOrganic active ingredientsDegenerative neurologic diseasesParenteral nutrition
The present invention involves the use of desmethyl tocopherols such as gamma tocopherol for the prevention of and treatment of neurological disorders. Dietary or parenteral administration of desmethyl tocopherols inhibits the undesired nitration of neurological components.
Owner:OKLAHOMA MEDICAL RES FOUND
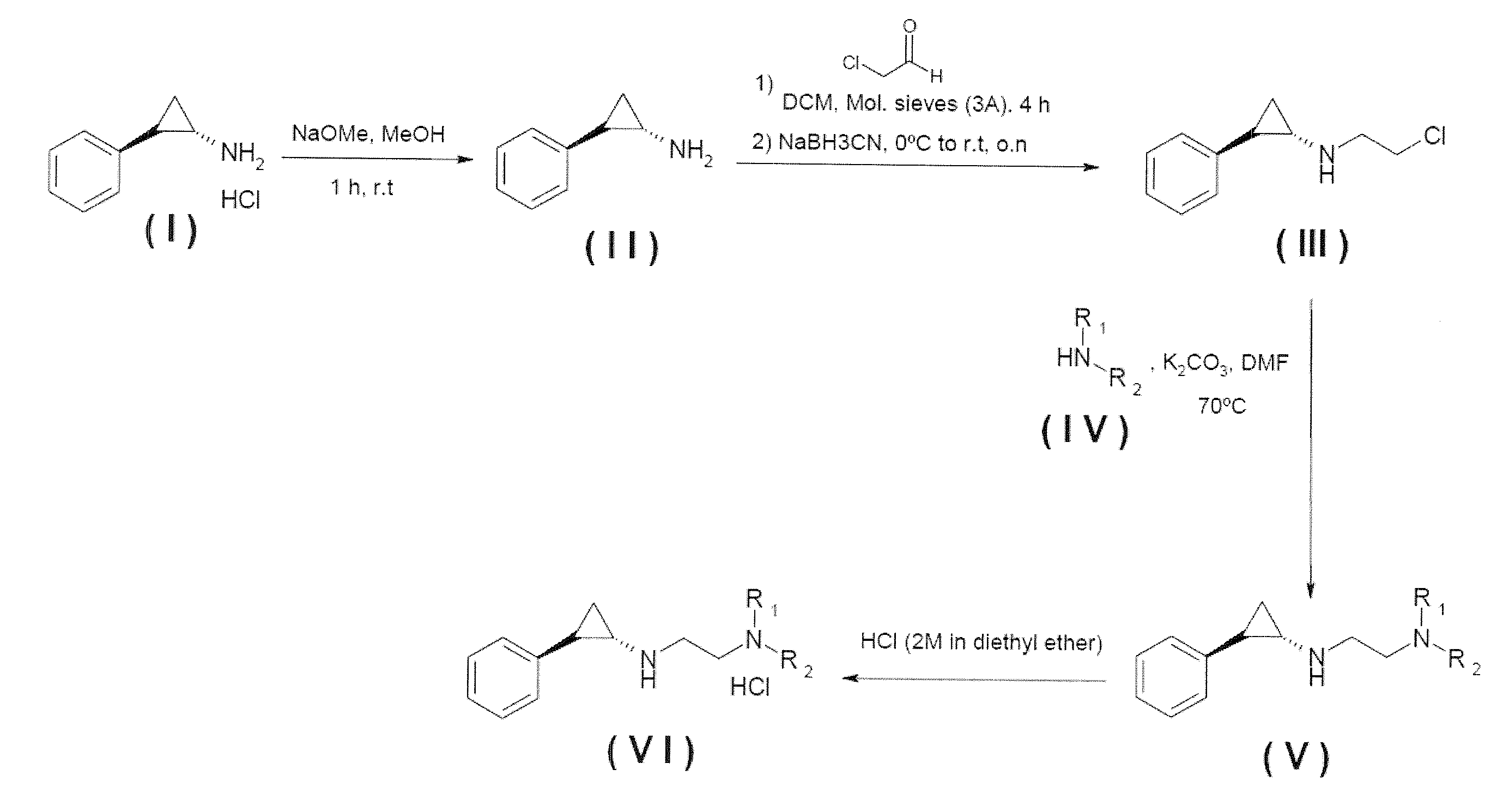
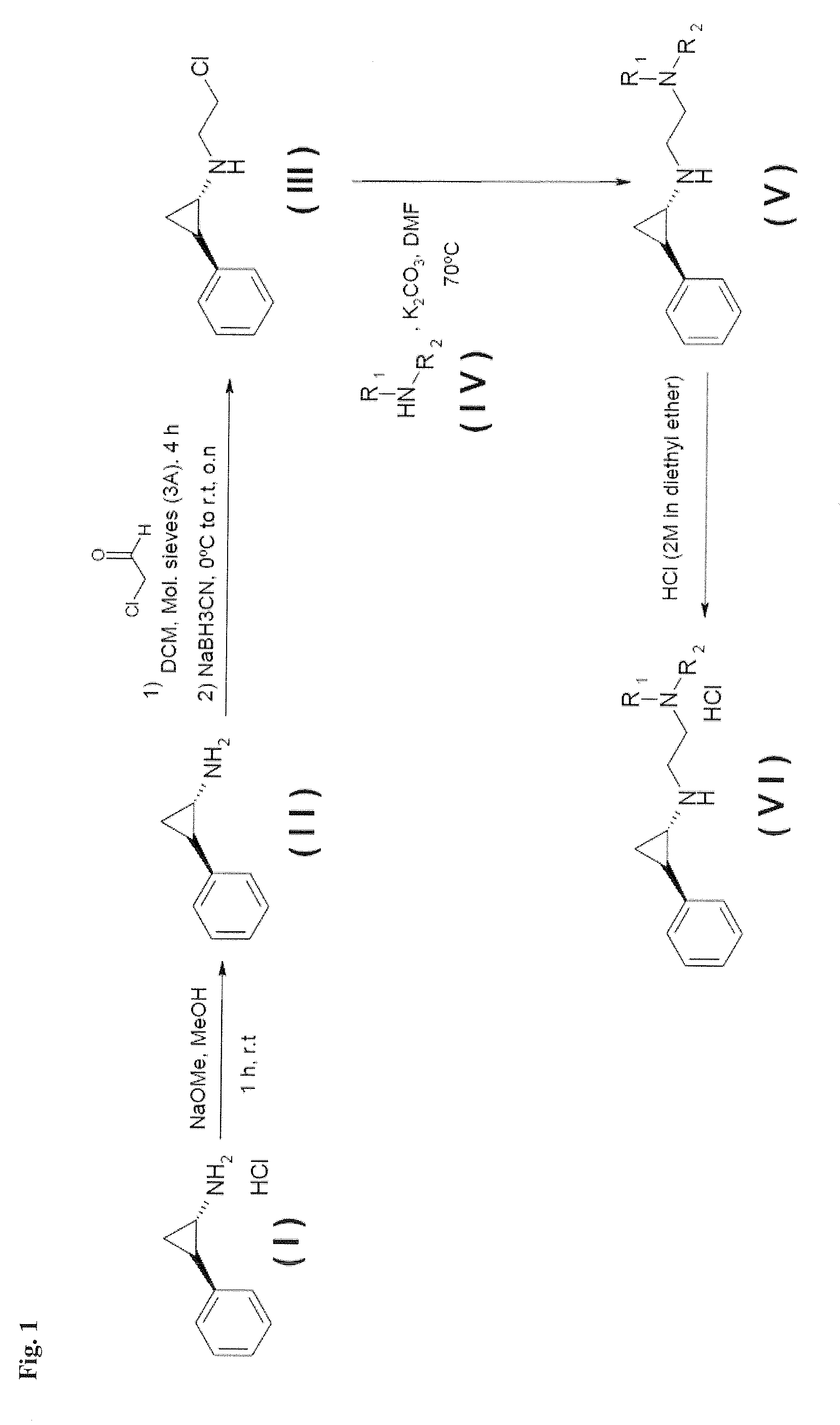
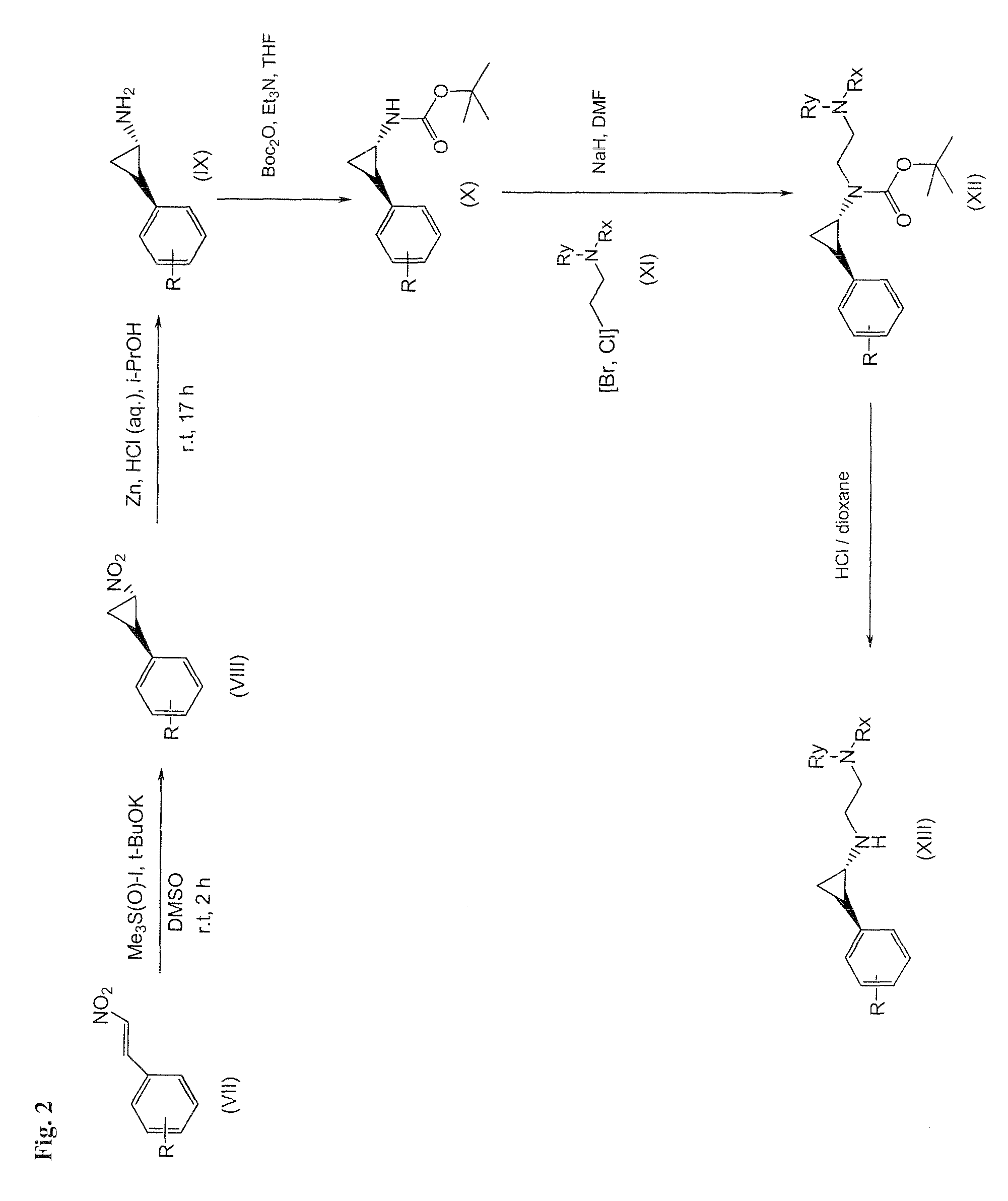
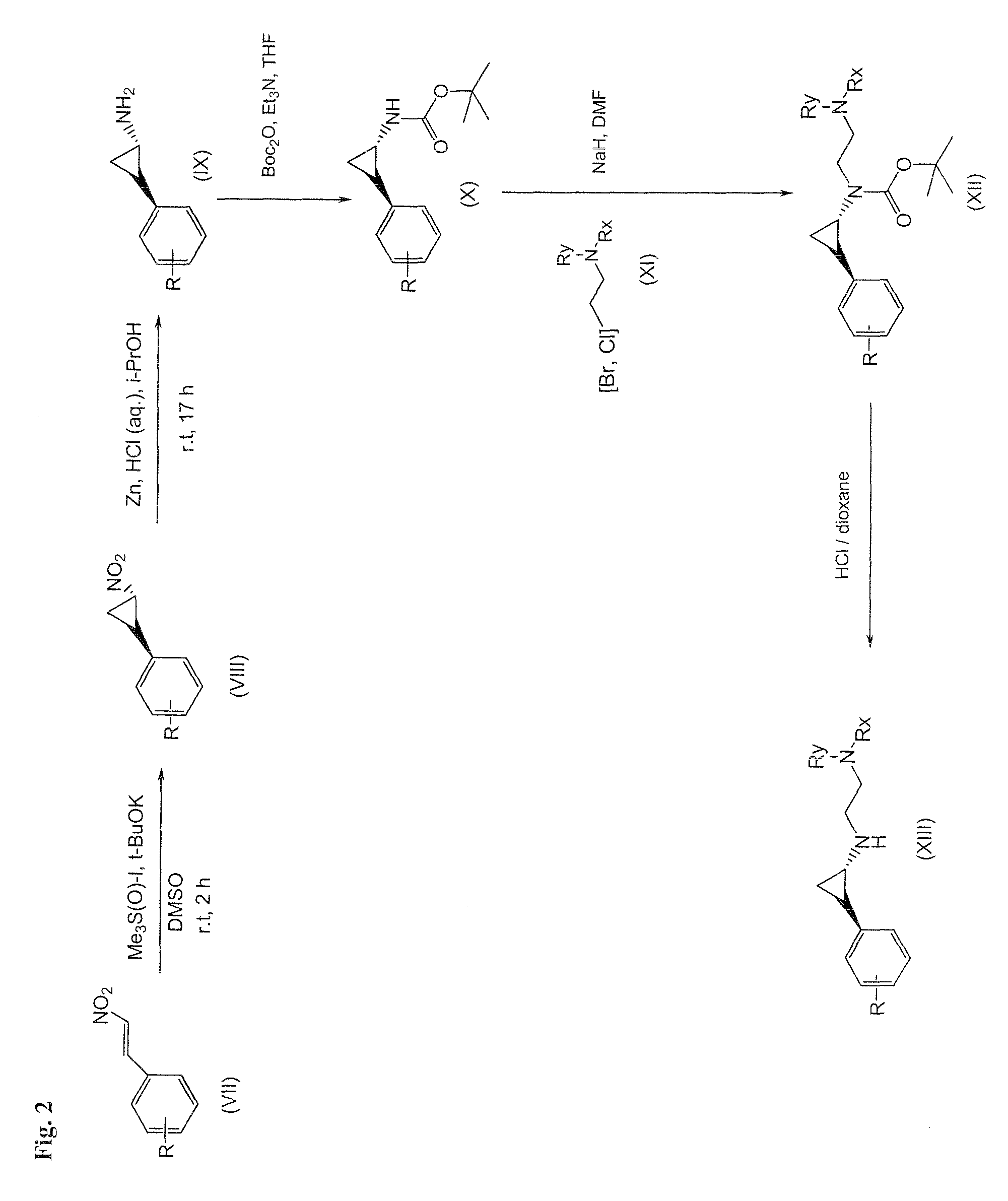
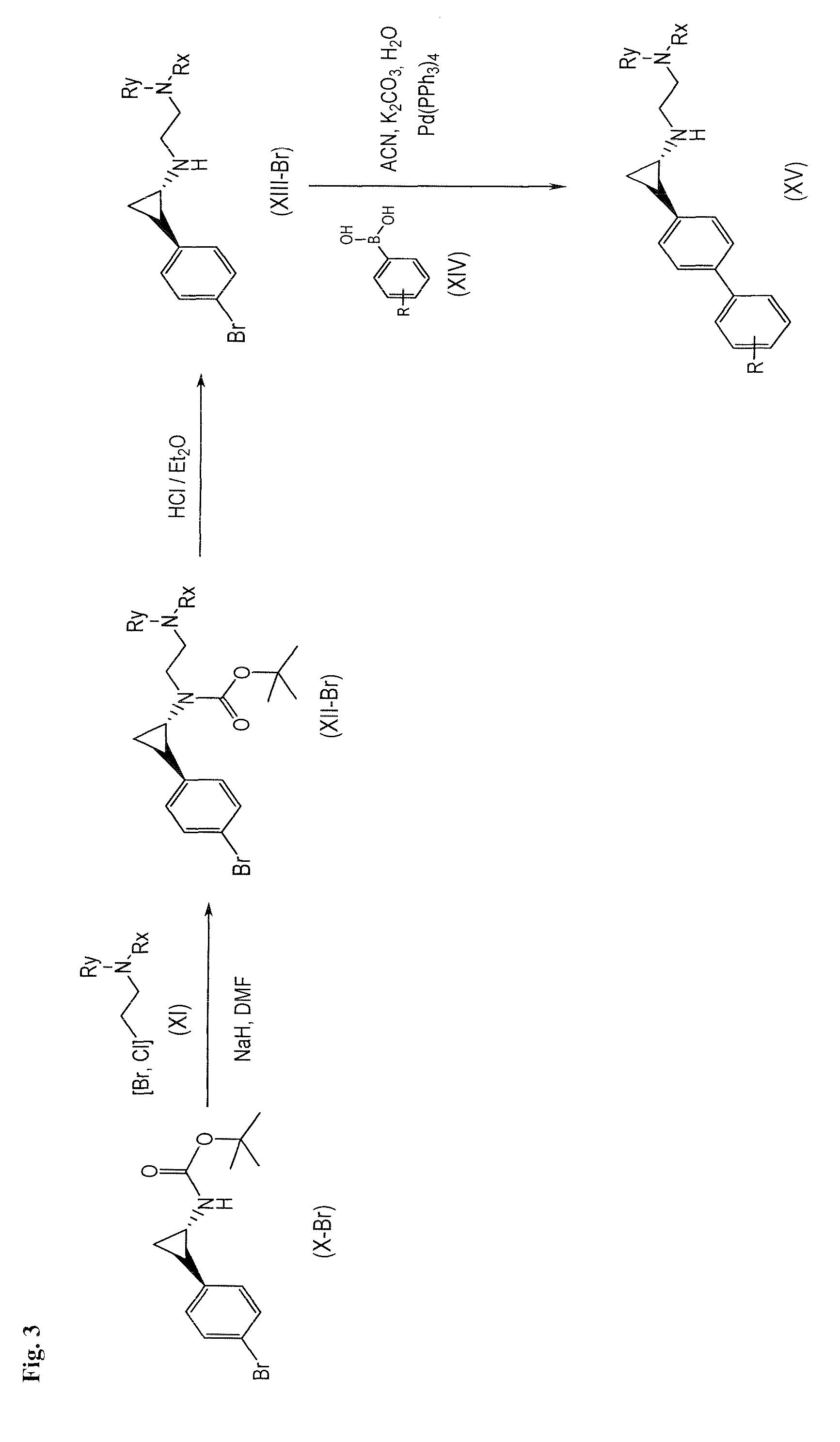
Arylcyclopropylamine based demethylase inhibitors of lsd1 and their medical use
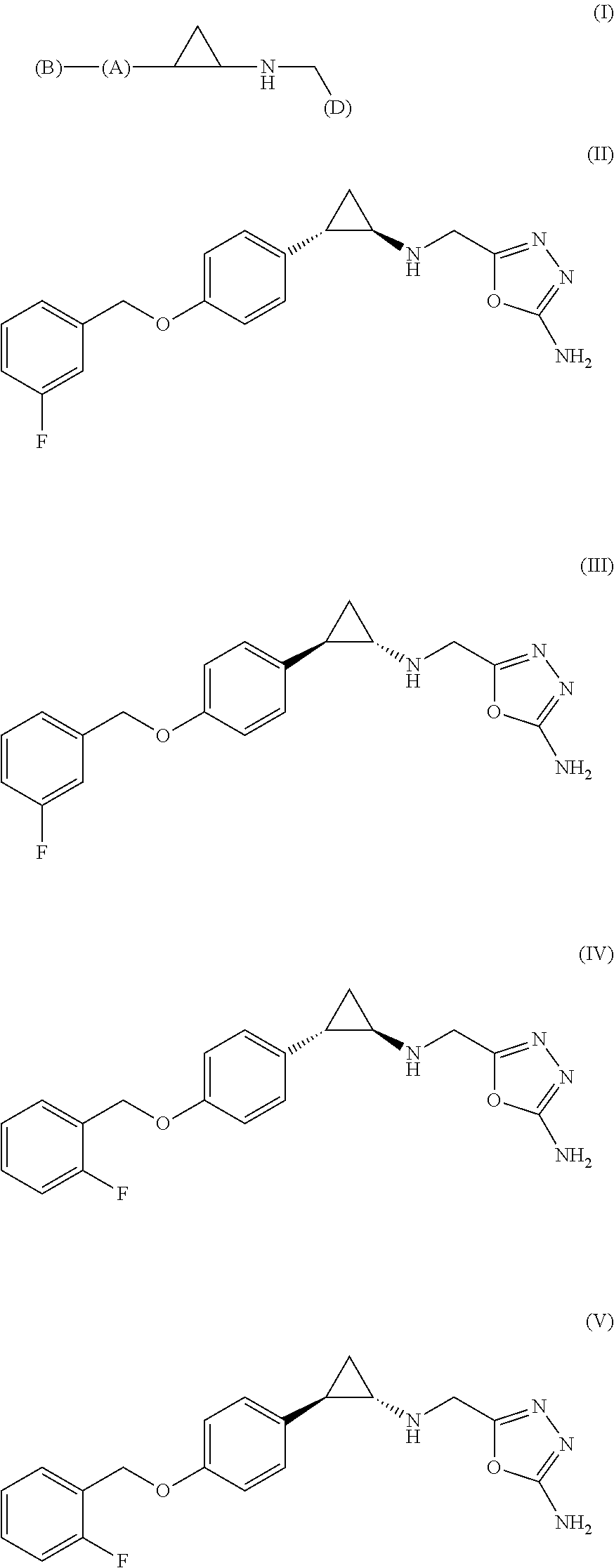
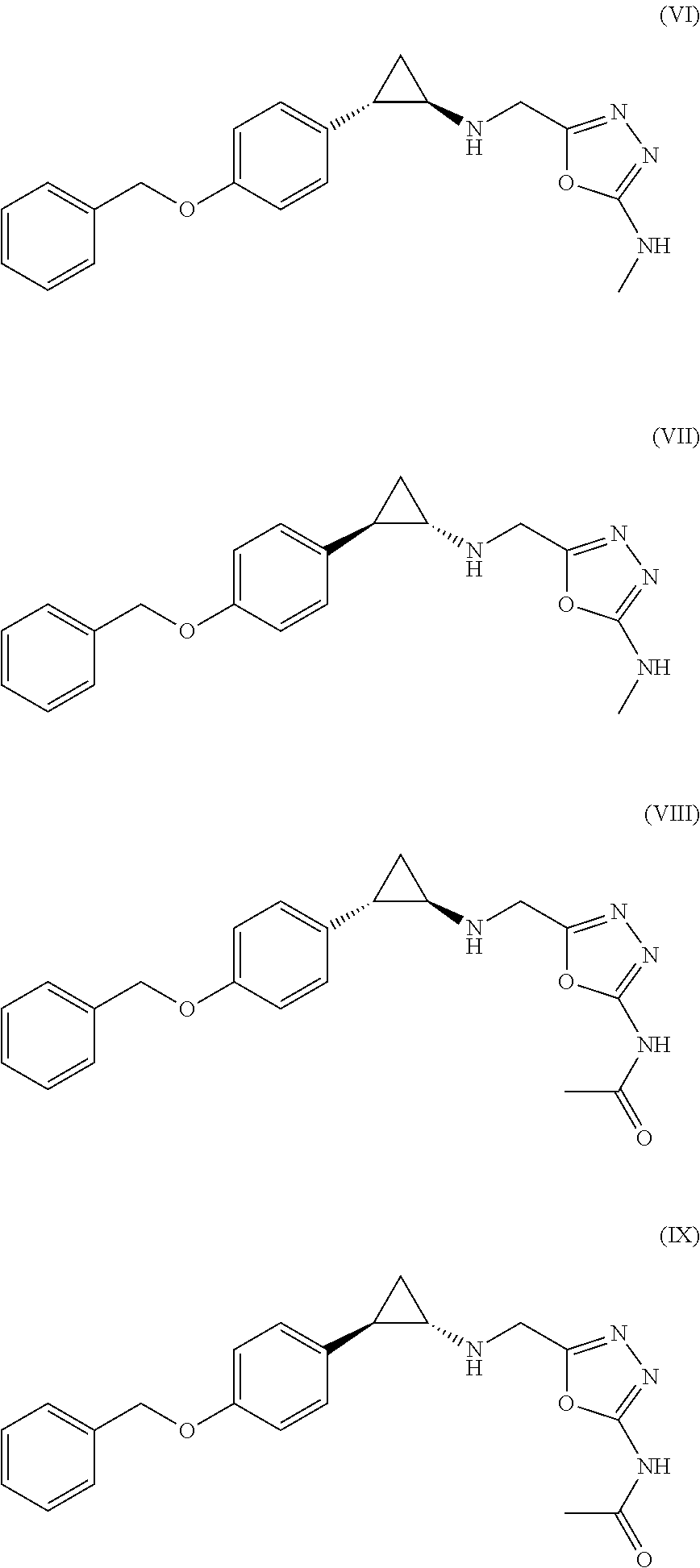
The invention relates to (hetero)aryl cyclopropylamine compounds, including particularly the compounds of formula (I) as described and defined herein, and their use in therapy, including, e.g., in the treatment or prevention of cancer, a neurological disease or condition, or a viral infection. Thus, in one specific aspect the invention relates to formulas (II), (III), (IV), (V), (VI), (VII), (VIII), (IX).
Owner:ORYZON GENOMICS SA
Desmethyl tocopherols for protecting cardiovascular tissue
InactiveUS20010044462A1Safe deliveryAmenable to deliveryOrganic active ingredientsBiocideThrombusNitrogen
The present invention involves the use of desmethyl tocopherols such as gamma tocopherol for the protection of cardiovascular tissue from nitrative stress. While mechanisms other than scavenging of reactive nitrogen species may be involved, desmethyl tocopherols exhibit significant protection and may be utilized to treat or help prevent cardiovascular particularly arterial vascular disease. The desmethyl tocopherols may be administered dietarily or parenterally when a more direct dosage is desired. Both routes may be utilized together or separately to optimize therapeutic and prophylactic benefits. The lessening of damage induced by reactive nitrogen species leads to the lessening of arterial blockage in thrombosis.
Owner:OKLAHOMA MEDICAL RES FOUND
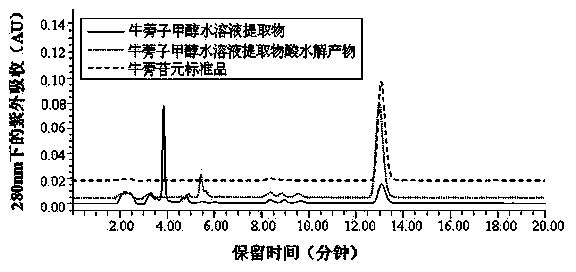
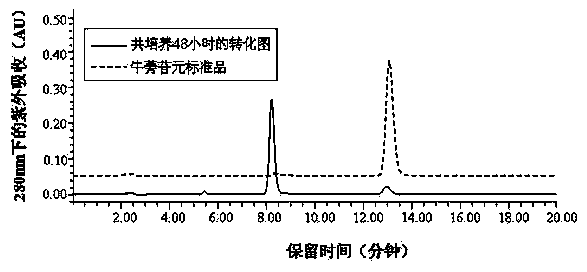
Blautia sp. AUH-JLD56 and application thereof in conversion of arctigenin
ActiveCN103509741ASolve insufficient resourcesBig pushBacteriaMicroorganism based processesMicroorganismActive matter
The invention discloses a blautia sp. AUH-JLD56 and an application thereof in the conversion of arctigenin, and belongs to the technical field of bacterium. The preservation number of blautia sp. AUH-JLD56 is CGMCC No. 8032. The application of blautia sp. AUH-JLD56 comprises following steps: (1) culture of bacterium strain AUH-JLD56; (2) co-culture of a substrate, namely a coarse product of arctigenin, and a bacterium strain AUH-JLD56 and separation and purification of metabolite. The bacterium strain AUH-JLD56 can high effectively convert a coarse product of arctigenin in the traditional Chinese medicine actium lappa to 3'-demethylated-arctigenin, solves the 3'-demethylated-arctigenin resource shortage problem, and lays a foundation for researching and developing microorganism metabolite of pharmacological active substance in actium lappa.
Owner:HEBEI AGRICULTURAL UNIV.
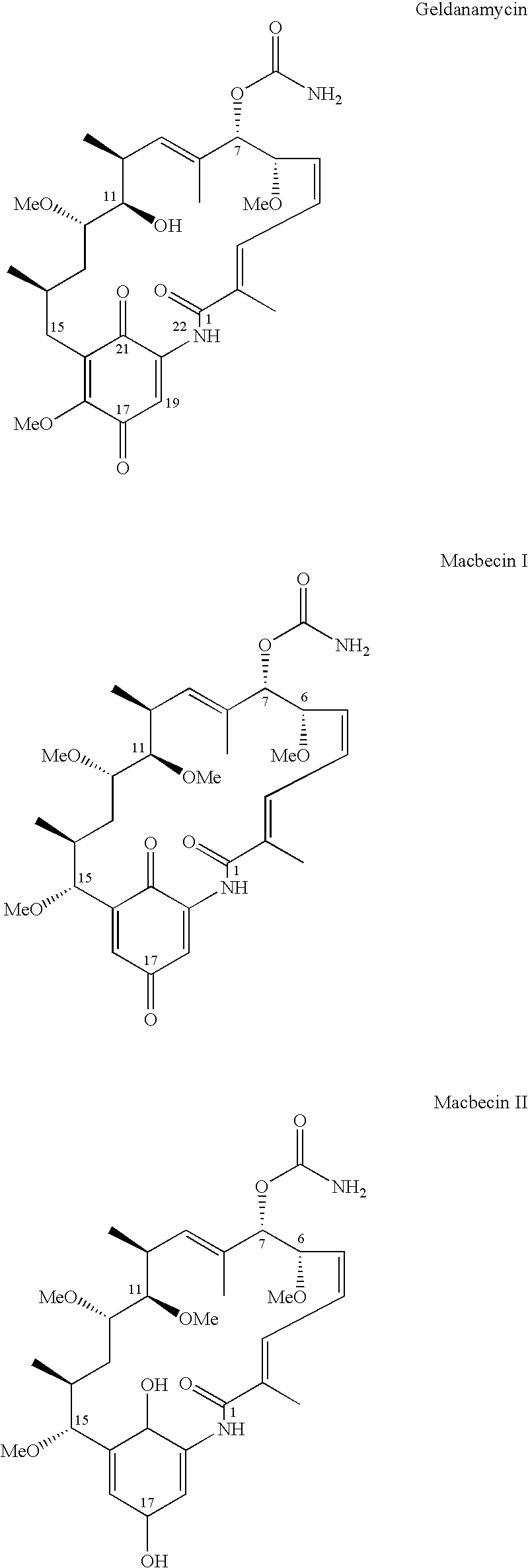
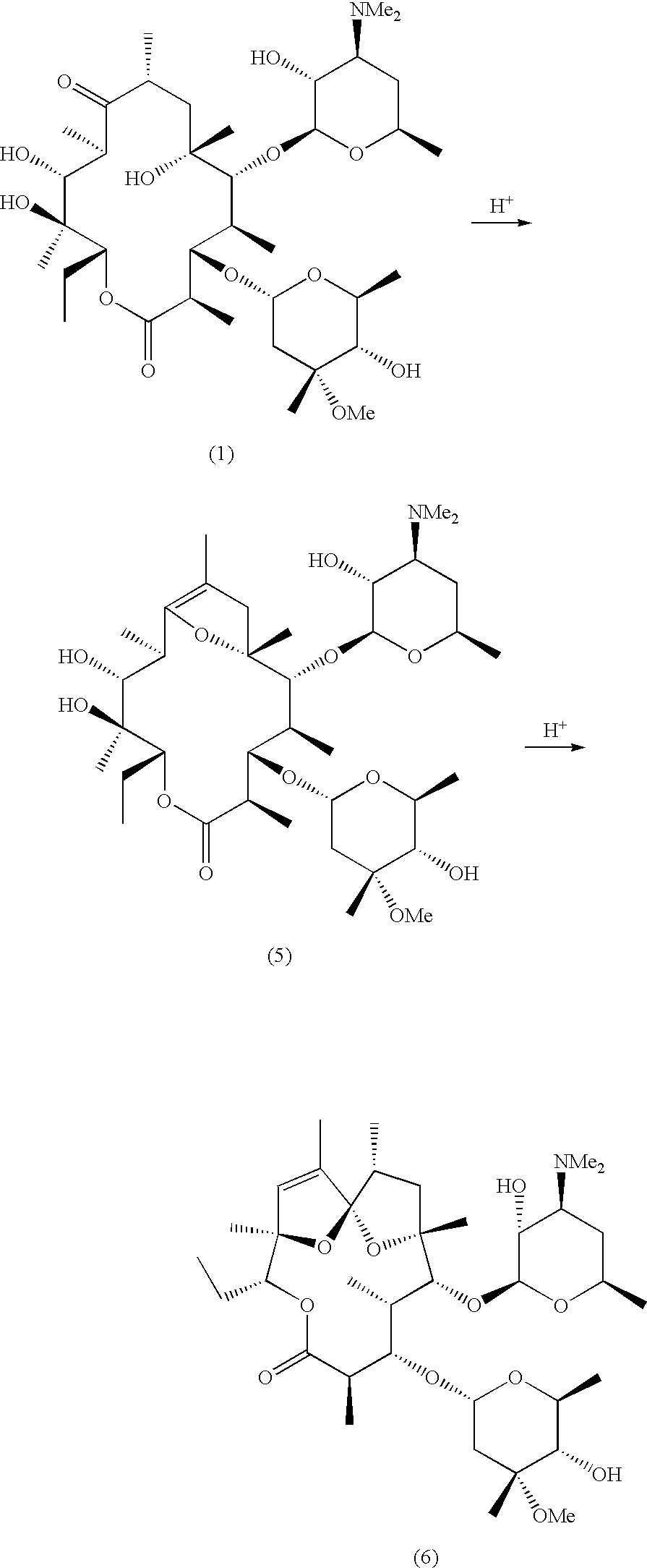
2-Desmethyl ansamycin compounds
ActiveUS20050026894A1Reduce potential side effectsBiocideOrganic chemistryAntiproliferative AgentsMedicinal chemistry
2-Desmethyl ansamycins having a structure according to formula I, where R1, R2, R3, R4, R5 and R6 are as defined herein, and other 2-desmethyl ansamycins are useful as antiproliferative agents.
Owner:KOSAN BIOSCI
Pharmaceutical combinations comprising cd33 antibodies and de-methylating agents
InactiveUS20150125447A1Enhanced ADCC activityADCC activityOrganic active ingredientsAntibody ingredientsDiseaseMethylating Agent
The present invention relates to pharmaceutical combinations CD33 antibodies and de-methylating agents for use in treating diseases like MDS and cancer, especially AML.
Owner:BOEHRINGER INGELHEIM INT GMBH
Succinate salt of O-desmethyl-venlafaxine
InactiveUS7026508B2Suitable for useImprove bioavailabilityOrganic active ingredientsNervous disorderSuccinatesDesmethyl
A novel salt of O-desmethyl venlafaxine is provided, O-desmethylvenlafaxine succinate. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH LLC
Blautia sp. AUH-JLD56 and application thereof in conversion of arctigenin
ActiveCN103509741BSolve insufficient resourcesBig pushBacteriaMicroorganism based processesMicroorganismMicrobial metabolite
The invention discloses a Brautieria AUH-JLD56 and its application in the transformation of arctigenin, belonging to the technical field of bacteria. Blautia sp. AUH-JLD56, the preservation number is CGMCCNo.8032. Its application includes the following steps: (1) cultivation of bacterial strain AUH-JLD56; (2) co-cultivation of substrate arctigenin crude product and bacterial strain AUH-JLD56 and separation and purification of metabolites. The bacterial strain of the present invention can efficiently convert the crude arctigenin in the traditional Chinese medicine Arctium Fructus into 3′-demethyl-arctigenin, which solves the problem of lack of 3′-demethyl-arctigenin resources, and is an effective method for The research and development of microbial metabolites of pharmacologically active substances has laid the foundation.
Owner:HEBEI AGRICULTURAL UNIV.
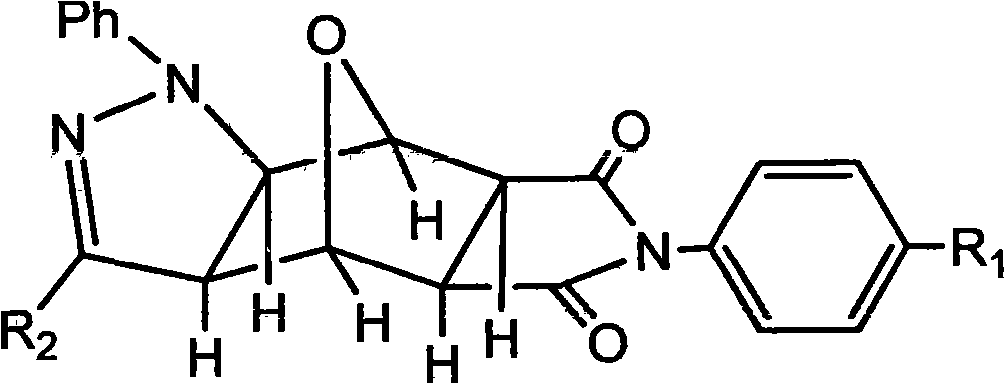
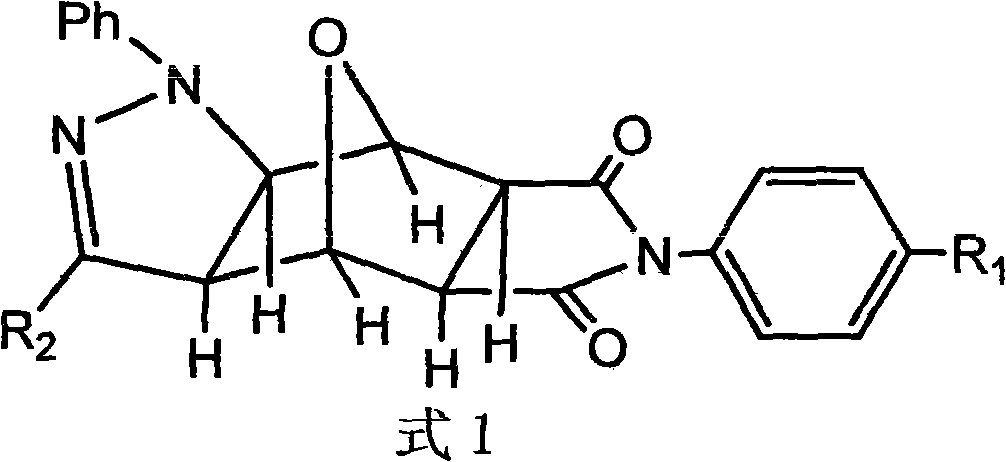
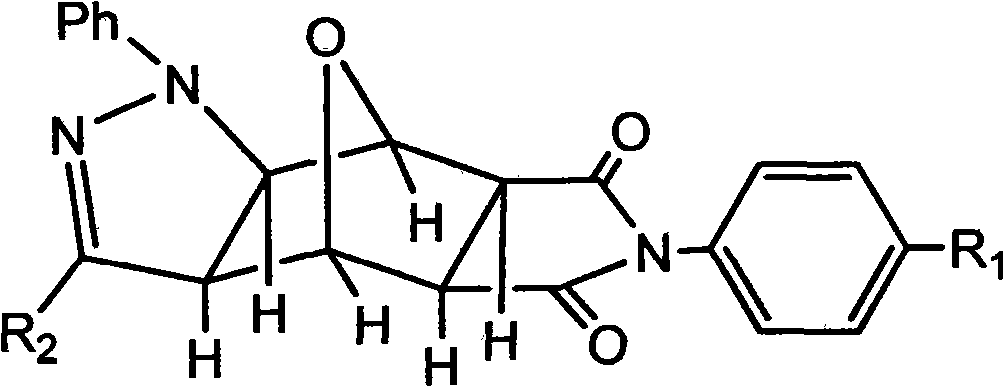
Pyrazolo N-substituted dehydronorcantharidin imide derivative as well as synthesis method, activity test method and application thereof
InactiveCN101812066AGood antitumor activityOrganic active ingredientsOrganic chemistryImideQuinoxaline
The invention discloses a pyrazolo N-substituted dehydronorcantharidin imide derivative as well as a synthesis method, an activity test method and application thereof, belonging to the field of cantharidin derivatives. The pyrazolo N-substituted dehydronorcantharidin imide derivative has a structural general formula shown as a formula 1: in the formula 1, R1 is H, C1, F, CH3, OCH3, OH or NO2; and R2 is 2-phenyl-2H-1,2,3-triazole-4-substituent or quinoxaline-2-substituent. The novel N-substituted dehydronorcantharidin imide derivative introduces five-membered heterocyclic pyrazole rings into norcantharidin substituted arylamine and has favorable anti-tumor activity.
Owner:SHAOXING UNIVERSITY
Lysine specific demethylase-1 inhibitors and their use
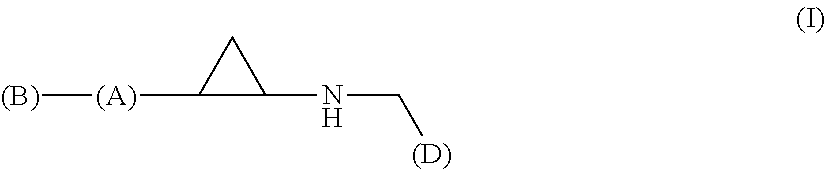
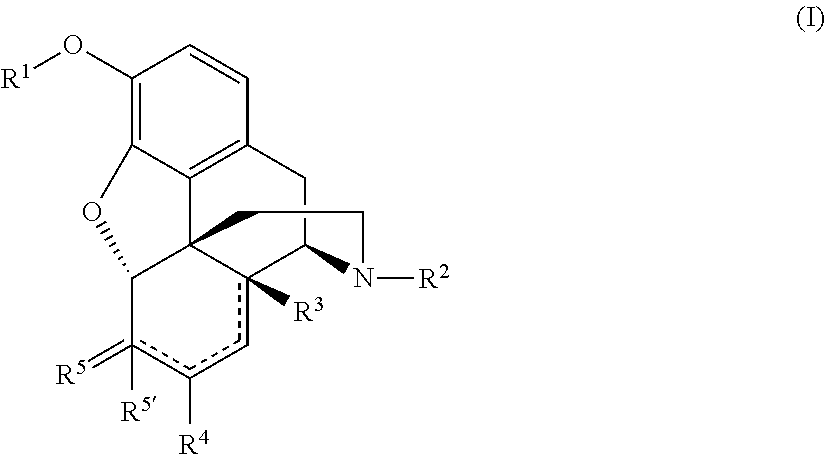
The invention relates to a compound of Formula (I): (A′)x-(A)-(B)-(Z)-(L)-(D), wherein: (A) is heteroaryl or aryl; each (A′), if present, is independently chosen from aryl, arylalkoxy, arylalkyl, heterocyclyl, aryloxy, halo, alkoxy, haloalkyl, cycloalkyl, haloalkoxy, and cyano, wherein each (A′) is substituted with 0, 1, 2, or 3 substituents independently chosen from halo, haloalkyl, aryl, arylalkoxy, alkyl, alkoxy, cyano, sulfonyl, amido, and sulfinyl; X is 0, 1, 2, or 3; (B) is a cyclopropyl ring, wherein (A) and (Z) are covalently bonded to different carbon atoms of (B); (Z) is —NH—; (L) is chosen from —CH2CH2—, —CH2CH2CH2—, and —CH2CH2CH2CH2—; and (D) is chosen from —N(—R1)—R2, —O—R3, and —S—R3, wherein: R1 and R2 are mutually linked to form a heterocyclic ring together with the nitrogen atom that R1 and R2 are attached to, wherein said heterocyclic ring has 0, 1, 2, or 3 substituents independently chosen from —NH2, —NH(C1-C6 alkyl), —N(C1-C6 alkyl)(C1-C6 alkyl), alkyl, halo, cyano, alkoxy, haloalkyl, and haloalkoxy, or R1 and R2 are independently chosen from —H, alkyl, cycloalkyl, haloalkyl, and heterocyclyl, wherein the sum of substituents on R1 and R2 together is 0, 1, 2, or 3, and the substituents are independently chosen from —NH2, —NH(C1-C6 alkyl), —N(C1-C6 alkyl)(C1-C6 alkyl), and fluoro; and R3 is chosen from —H, alkyl, cycloalkyl, haloalkyl, and heterocyclyl, wherein R3 has 0, 1, 2, or 3 substituents independently chosen from —NH2, —NH(C1-C6 alkyl), —N(C1-C6 alkyl)(C1-C6 alkyl), and fluoro; or an enantiomer, diastereomer, or mixture thereof, or a pharmaceutically acceptable salt or solvate thereof. The compounds of the invention show inhibitory LSD1 activity, which makes them useful in the treatment or prevention of diseases such as cancer.
Owner:ORYZON GENOMICS SA
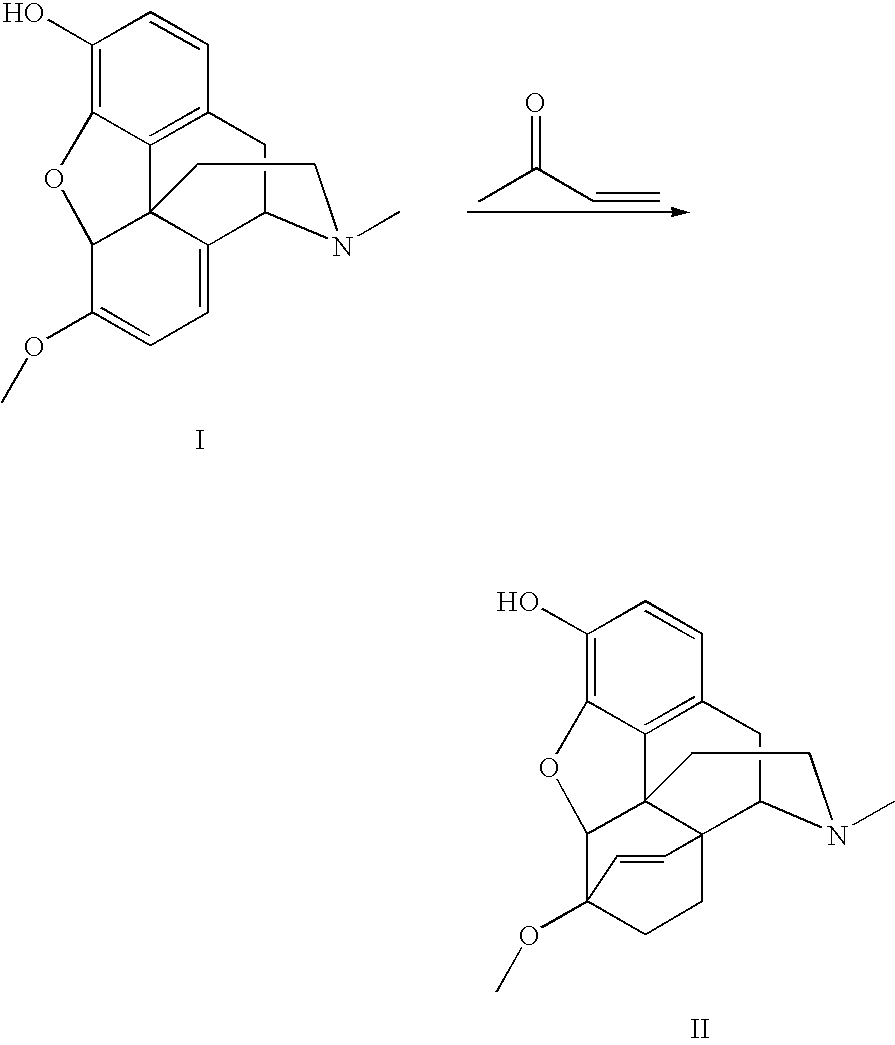
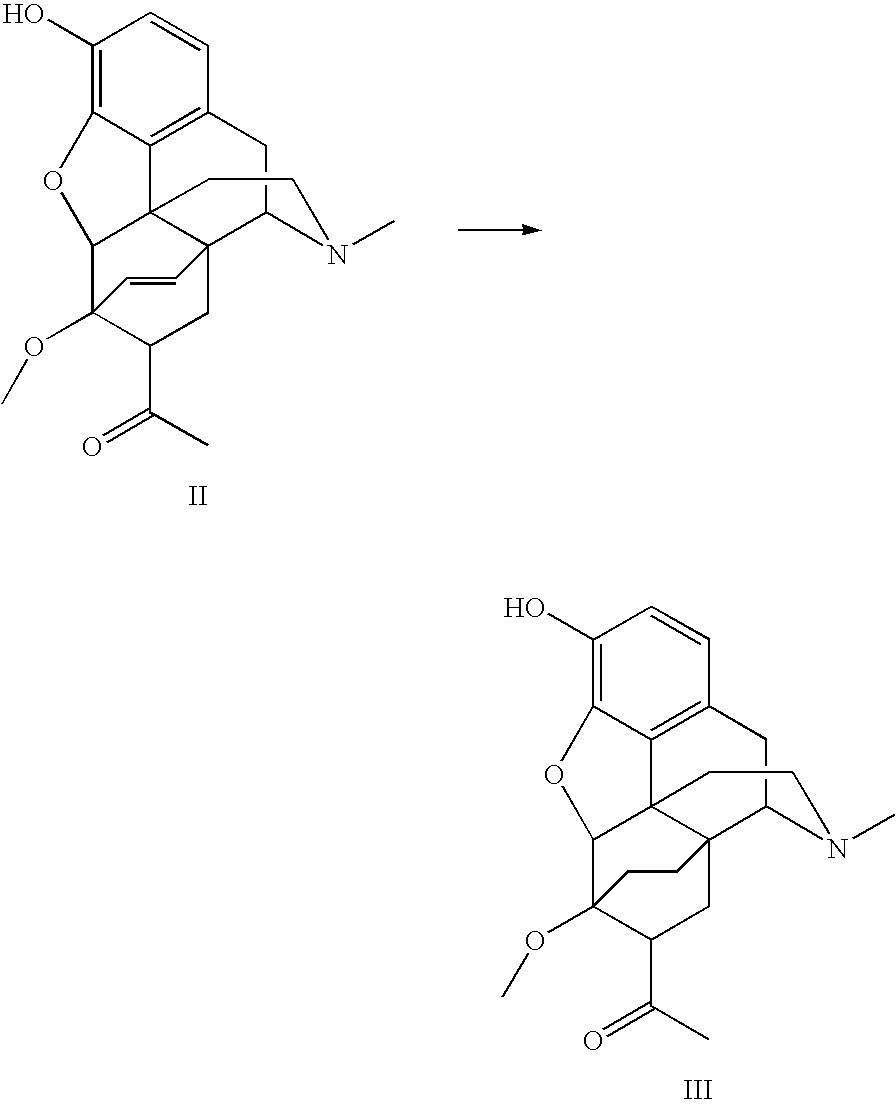
Methods for One-Pot N-Demethylation/N-Functionalization of Morphine and Tropane Alkaloids
InactiveUS20110313163A1Safe and cost-effectiveReduce the temperatureOrganic chemistryMorphineTropane alkaloid
The present invention provides a method for the N-demethylation and N-functionalization of an N-methylated heterocycle such as a morphine alkaloid or tropane alkaloid. The method comprises reacting the heterocycle with an functionalization agent in the presence of a transition metal catalyst in air or in the presence of an oxidant.
Owner:BROCK UNIVERSITY
Extended release pharmaceutical dosage form
This invention relates to novel extended release pharmaceutical dosage forms for orally delivering drugs to mammals, e.g., humans. More particularly, this invention concerns novel dosage forms of water soluble drugs such as venlafaxine, enantiomeric (R or S) forms of venlafaxine, metabolites of venlafaxine such as O-desmethyl venlafaxine (ODV) or enantiomeric (R or S) forms of said metabolites which dosage forms have an extended release profile when taken orally. This invention also provides processes for preparing such dosage forms and methods of using them.
Owner:WYETH LLC
Lysine specific demethylase-1 inhibitors and their use
The present invention relates to a compound of Formula 1, wherein: (A) is heteroaryl or aryl; each (A′), if present, is independently chosen from aryl, arylalkoxy, arylalkyl, heterocyclyl, aryloxy, halo, alkoxy, haloalkyl, cycloalkyl, haloalkoxy, and cyano, wherein each (A′) is substituted with 0, 1, 2, or 3 substituents independently chosen from halo, haloalkyl, haloalkoxy, aryl, arylalkoxy, alkyl, alkoxy, amido, —CH2C(=0)NH2, heteroaryl, cyano, sulfonyl, and sulfinyl; X is 0, 1, 2, or 3; (B) is a cyclopropyl ring, wherein (A) and (Z) are covalently bonded to different carbon atoms of (B); (Z) is —NH—; (L) is chosen from a single bond, —CH2—, —CH2CH2—, —CH2CH2CH2—, and —CH2CH2CH2CH2—; and (D) is an aliphatic carbocyclic group or benzocycloalkyl, wherein said aliphatic carbocyclic group or said benzocycloalkyl has 0, 1, 2, or 3 substituents independently chosen from —NH2, —NH(C1-C6 alkyl), —N(C1-C6 alkyl)(C1-C6 alkyl), alkyl, halo, amido, cyano, alkoxy, haloalkyl, and haloalkoxy. (A′)X-(A)-(B)—(Z)-(L)-(D) formula (I) The compounds of the invention show activity for inhibiting LSD1, which makes them useful in the treatment or prevention of diseases such as cancer.
Owner:ORYZON GENOMICS SA
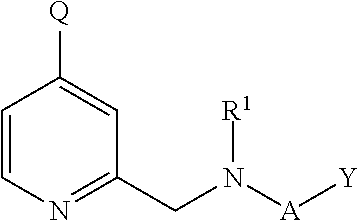
N-desmethyl-doxepin and methods of using the same to treat sleep disorders
Owner:PROCOM ONE +1
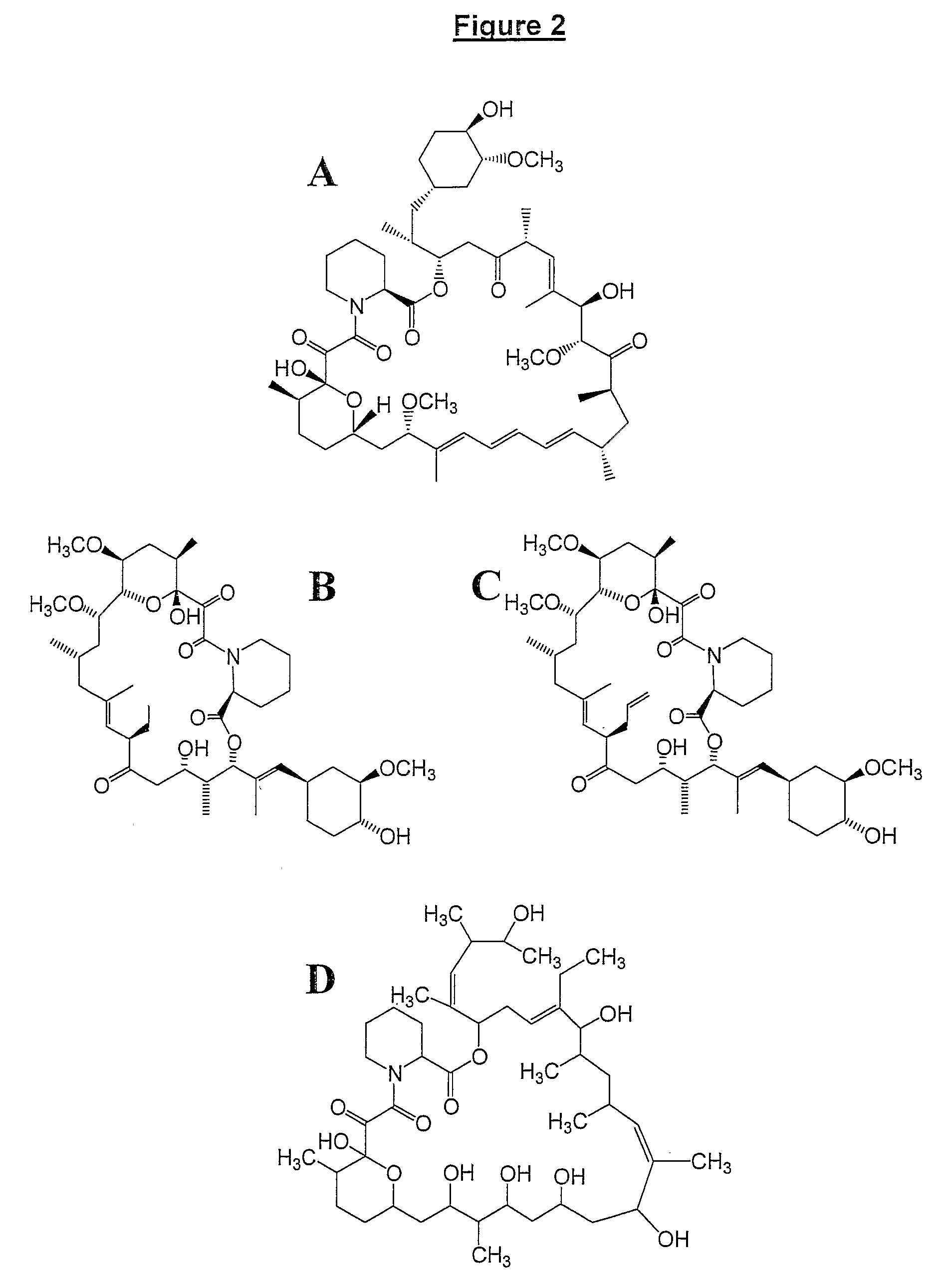
Production Of Polyketides And Other Natural Products
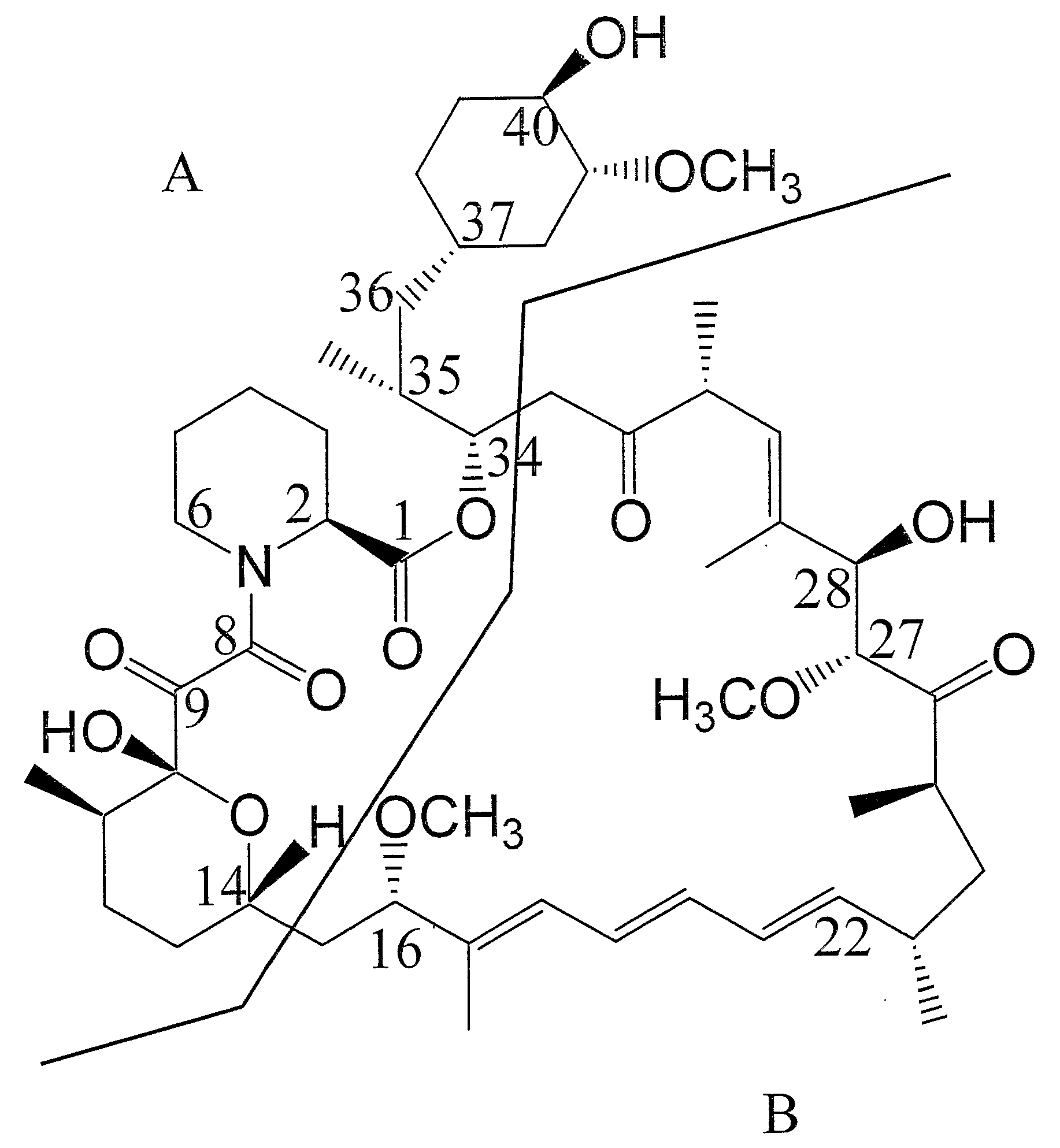
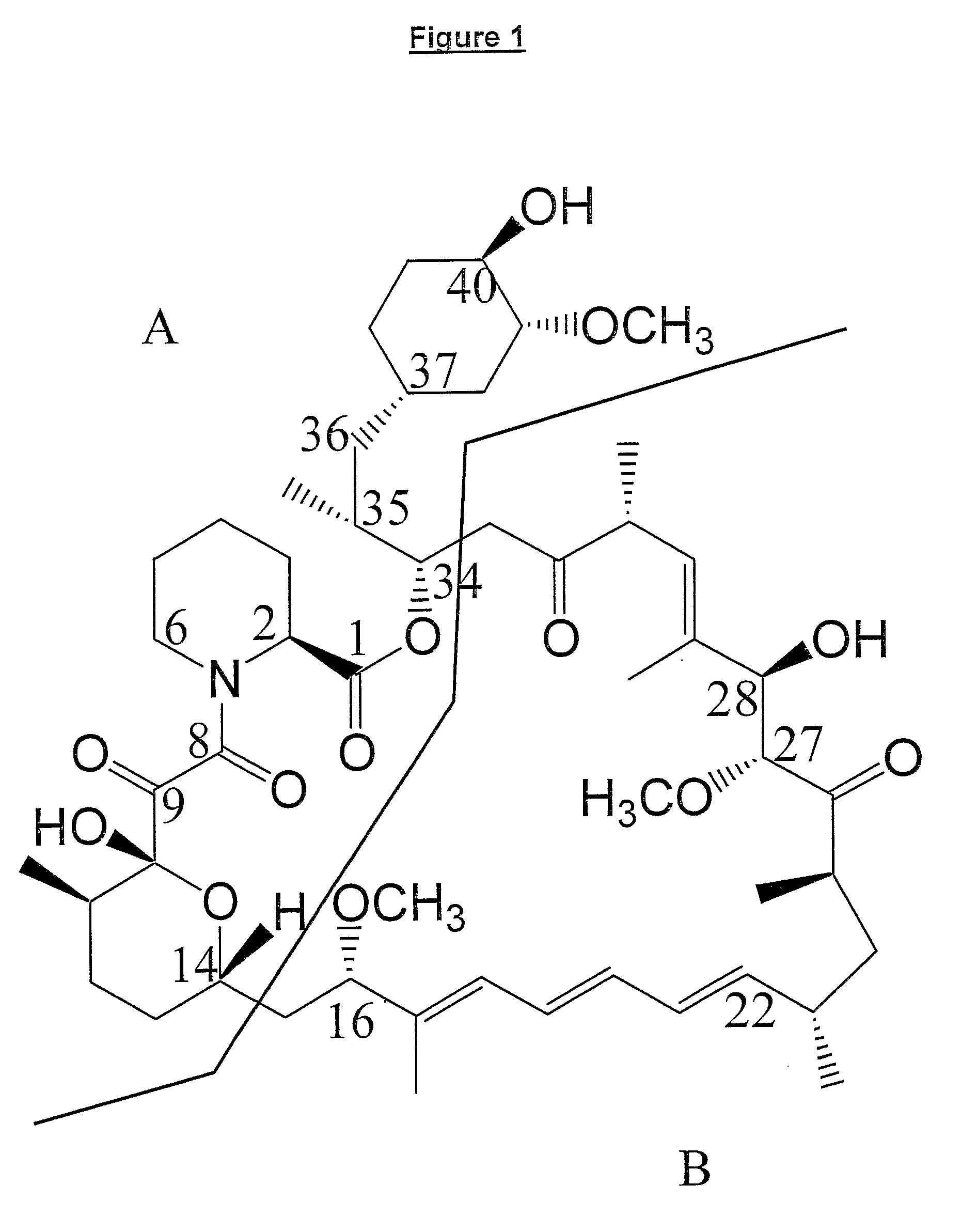
The present invention relates to production of polyketides and other natural products and to libraries of compounds and individual novel compounds. Therefore in aspect the present invention provides 17-desmethylrapamycin and analogues thereof, methods for their production, including recombinant strains, and isolation and uses of the compounds of the invention. In a further aspect the present invention provides for the use of 17-desmethylrapamycin and analogues thereof in the induction or maintenance of immunosuppression, the stimulation of neuronal regeneration or the treatment of cancer, B-cell malignancies, fungal infections, transplantation rejection, graft vs. host disease, autoimmune disorders, diseases of inflammation vascular disease and fibrotic diseases, and in the regulation of wound healing.
Owner:BIOTICA TECH
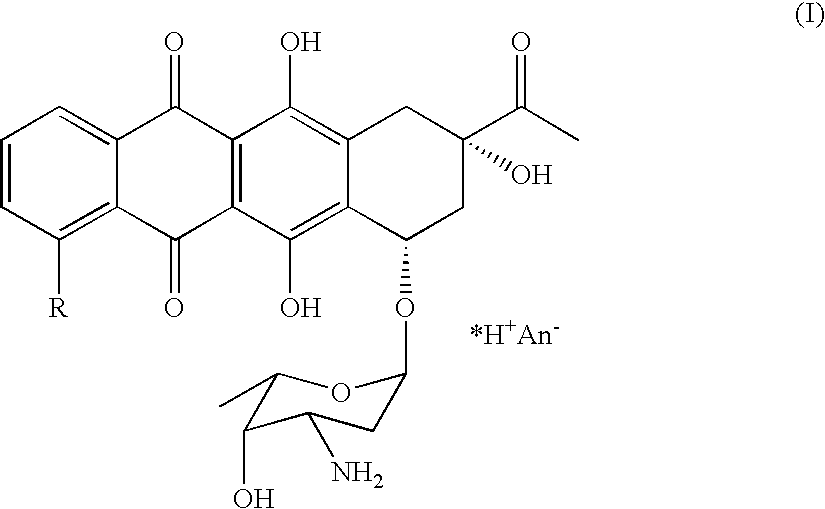
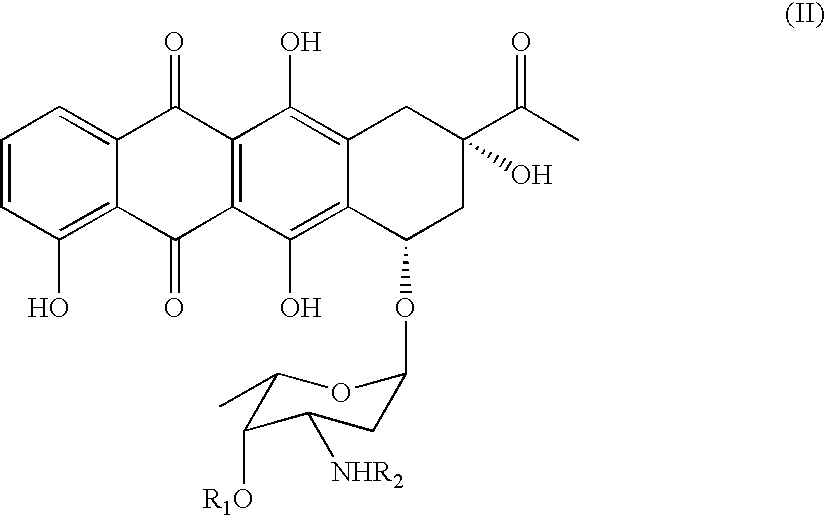
Method of preparing 4-R-substituted 4-demethoxydaunorubicin
ActiveUS7053191B2Reduce in quantityImprove processing yieldSugar derivativesSugar derivatives preparationPtru catalystAcyl group
A method of synthesizing 4-R-substituted anthracyclines and their corresponding salts from 4-demethyldaunorubicin includes the steps of treating 4-demethyldaunorubicin with a sulfonylating agent to form 4-demethyl-4-sulfonyl-R3-daunorubicin. 4-Demethyl-4-R3-sulfonyl-daunorubicin is then subject to a reducing agent in the presence of a transition metal catalyst in a temperature range of about 30° C. to about 100° C. in a polar aprotic solvent in an inert atmosphere. Protected 4-demethoxy-4-R-daunomycin then undergoes hydrolysis in a basic solution to form the 4-R-substituted anthracyclines. The novel method lacks the step of forming a stereospecific glycoside bond between aglycone and aminoglycoside. The method also increases the yield of the final product up to 30 to 40%.
Owner:SYNBIAS PHARMA
Macrocyclic and cage-like molecules based on biphenylarene and derivatives of macrocyclic and cage-like molecules as well as synthetic methods and applications of macrocyclic and cage-like molecules and derivatives
ActiveCN110642684AAchieving Modular SynthesisEasy to take offIon-exchanger regenerationOrganic chemistry methodsPorphyrinPhenyl group
The invention discloses macrocyclic and cage-like molecules based on biphenylarene and derivatives of the macrocyclic and cage-like molecules as well as synthetic methods and applications of the macrocyclic and cage-like molecules and the derivatives. A series of novel macrocycles are obtained mainly by performing a reaction on bis(2,4-dialkoxyphenyl)arenes (naphthalene, anthracene, pyrene, porphyrin and the like) or tris(2,4-dialkoxyphenyl)arenes (benzene, sym-tribenzobenzene) and paraformaldehyde under the catalysis of a Lewis acid in high yield. In addition, perhydroxybiphenylarenes (quaterphenyl trimer, naphthalene dimer and the like) can be obtained by performing demethylation, a plurality of water-soluble derivatives can be obtained by performing further modification, and the derivatives show good bonding ability to guest molecules (viologen and the like); and moreover, functional groups introduced into the framework make the biphenylarene have excellent adsorption and separationability and photophysical properties. The compounds, derivatives and methods provided by the invention have the following advantages: raw materials of the biphenylarene are commercially available, the synthesis is simple and convenient, the yield is high, and the modification is convenient, so that the compounds and the derivatives have broad application prospects in gas adsorption and separation, performance improvement of light-emitting materials, and adsorption of water-soluble toxic substances.
Owner:TIANJIN NORMAL UNIVERSITY
N-desmethyl-N-substituted-11-deoxyerythromycin compounds
ActiveUS20050119195A1Superior agonist activityLow antibacterial activityBiocideSugar derivativesMedicineMethyl group
Compounds having the structure of formula I wherein R1, R2, R3, R4, R5, and R6 are as defined herein, are prokinetic agents and can be used to treat disorders of gastric motility.
Owner:KOSAN BIOSCI
Cyclopropylamine spiro(hetero)cyclic compound, and pharmaceutical composition and application thereof
The invention relates to a preparation method for a cyclopropylamine spiro(hetero)cyclic compound and a composition containing the same, and application of the compound and the composition as an inhibitor for human lysine-specific demethylase (LSD1). The inhibitor is the cyclopropylamine spiro(hetero)cyclic compound as shown in a formula (I) which is described in the specification, or a pharmaceutically acceptable salt, predrug, solvate, polymorphic crystal or stable isotope derivative thereof. The compound can be used for treating or preventing diseases related to human lysine-specific demethylase, e.g., cancers and nerve diseases.
Owner:SHANGHAI DE NOVO PHARMA
Novel succinate salt of O-desmethyl-venlafaxine
InactiveUS20050096479A1Improve solubilityImprove permeabilityOrganic active ingredientsNervous disorderSuccinatesDesmethyl
A novel salt of O-desmethyl venlafaxine is provided, O-desmethylvenlafaxine succinate. Pharmaceutical compositions, dosage forms and methods of use are also provided.
Owner:WYETH
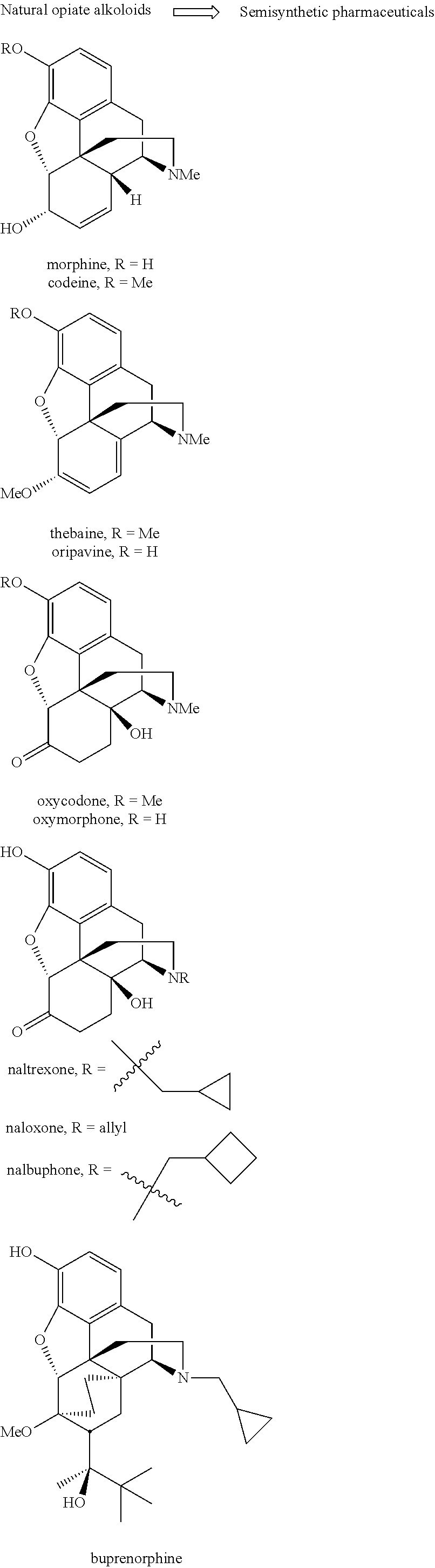
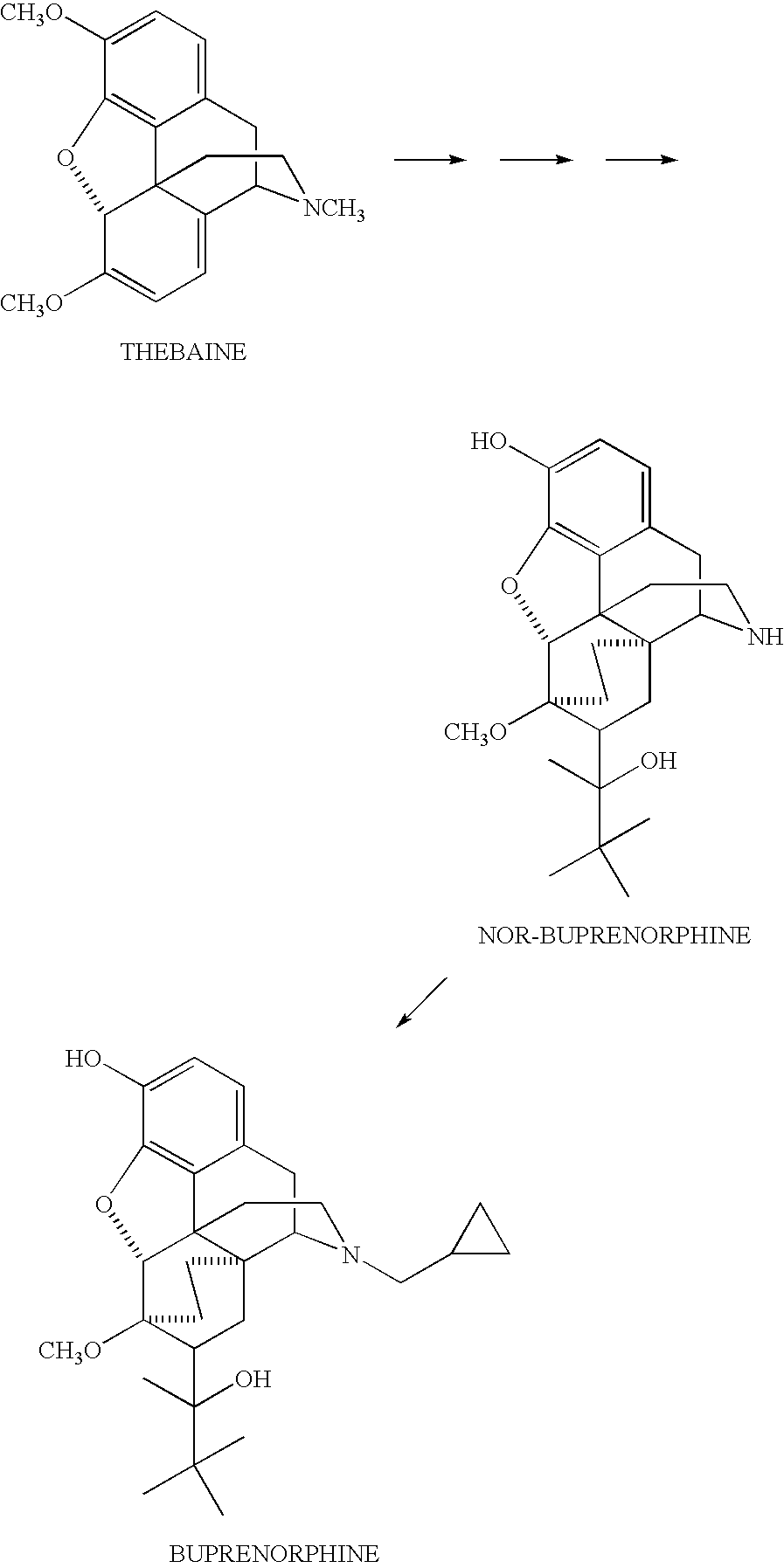
Use of Oripavine as a Starting Material For Buprenorphine
There is provided a method for the synthesis of norbuprenorphine, and ultimately buprenorphine, utilizing oripavine as the starting material. Conventional methods of producing buprenorphine utilize thebaine as the starting material, requiring an O-demethylation step, typically a low to moderate yield transformation. The present use of oripavine as a starting material does not require an O-demethylation step, since the oripavine molecule lacks an O-3 methyl group.
Owner:SPECGX LLC
Pyrimidine and triazole containing LSD1 inhibitor and preparation method and application thereof
ActiveCN106432248AEnhanced inhibitory effectReasonable synthetic designOrganic chemistryAntineoplastic agentsCancer cellEnzyme inhibition
The invention belongs to the field of medicinal chemistry, and discloses a pyrimidine and triazole containing compound and a preparation method and application thereof in preparation of anti-cancer medicine with lysine specific demethylase 1 (LSD1) being a target. The general formula of the compound is shown in the drawing I. In-vitro LSD1 enzyme inhibition activity experiments prove that by inhibiting LSD1 activity, the compound has obvious inhibiting and killing effects on kinds of cancer cells and can be applied to preparation of the anti-cancer medicine as a further developed lead compound.
Owner:ZHENGZHOU UNIV
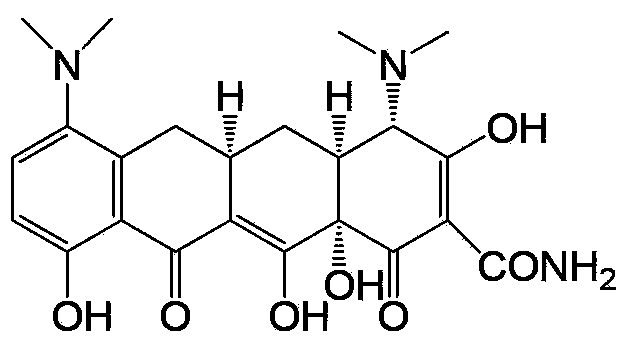
Preparation method and intermediate of minocycline
ActiveCN103387512ALow priceEasy to operateOrganic compound preparationCarboxylic acid amides preparationAlcoholPalladium
The invention discloses a preparation method and an intermediate of minocycline. A preparation method for the intermediate M-M is to subject demethylated aureomycin and dimethylamine to reactions as described in the specification in an amine solvent or amide solvent under the catalysis of a palladium complex. The preparation method for minocycline comprises the following steps: (1) subjecting demethylated aureomycin and dimethylamine to the reactions in the amine solvent or amide solvent under the catalysis of the palladium complex so as to prepare the compound M-M; and (2) subjecting the compound M-M prepared in step (1) to hydrogenation and dehydroxylation in an alcohol solvent including an acid under the catalysis of a catalyst. The preparation method provided by the invention has the advantages of easily available raw materials, low cost, simple two-step reaction operation, high product yield, good product quality, recoverability and reusability of the solvents and easy industrial production.
Owner:CHENGDU CHEMPARTNER
Methods for one-pot n-demethylation/n-acylation of morphine and tropane alkaloids
The present invention provides a method for the N-demethylation and / or N-acylation of an N-methylated heterocycle such as morphine alkaloids or tropane alkaloids. The method comprises reacting the heterocycle with an acylating agent in the presence of a metal catalyst.
Owner:BROCK UNIVERSITY
Lysine Specific Demethylase-1 inhibitors and their use
Owner:ORYZON GENOMICS SA
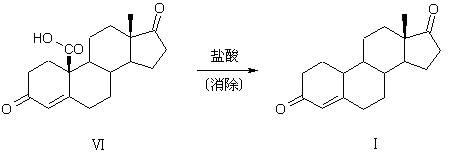
Preparation method of compound 19-desmethyl-4-androstene-3,17 diketone
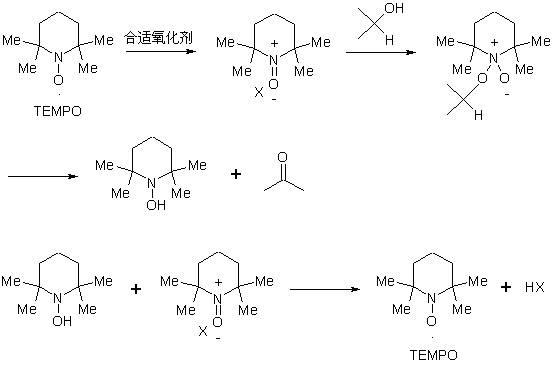
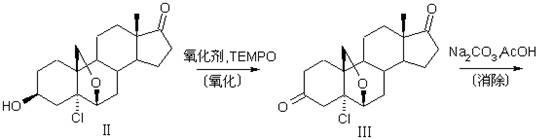
The invention discloses a preparation method of a compound 19-desmethyl-4-androstene-3,17 diketone, comprises the following step of by taking compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as raw materials, and carrying out oxidation reaction on the compounds of 5alpha-chloro-3beta-hydroxy-6beta and 19beta-epoxy-androstane-17-ketone as well as an intermediate compound 19-hydroxy-4-androstene-3,17-diketone with N-halogenated amide oxidant in a mixed solution of an organic solvent and an alkaline buffer solution in the presence of a catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide (TENPO) in the mild condition. In the two steps of oxidation reaction, the catalytic amount of 2,2,6,6-tetramethylpiperidine-N-oxide and the N-halogenated amide oxidant are both adopted to replace a mixed solution of chromium trioxide, sulfuric acid and water. In the oxidation method, without using the chromium trioxide, the use of carcinogenic substances are avoided being used, limitation by environmental protection is avoided and a great amount of wastes containing heavy metals in preparation are avoided being generated and accumulated, thereby no money and labor is consumed for removing the wastes.
Owner:ZHEJIANG XIANJU PHARMA
Prodrugs of muscarinic agonists and methods of treatment of neuropsychiatric disorders
Compounds are described that are prodrugs to active compounds that modulate a muscarinic receptor. In some cases, the compounds are prodrugs to N-desmethylclozapine. The compounds may be used to treat neuropsychiatric disorders.
Owner:ACADIA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com