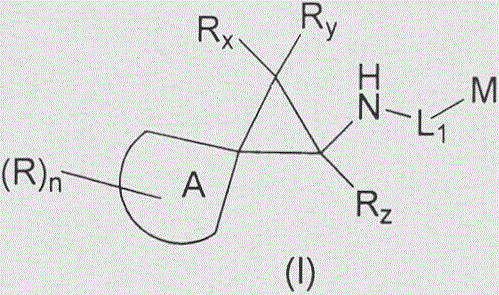
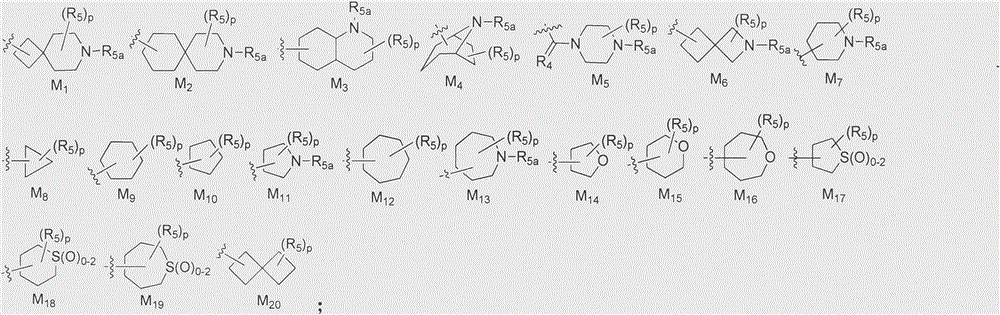
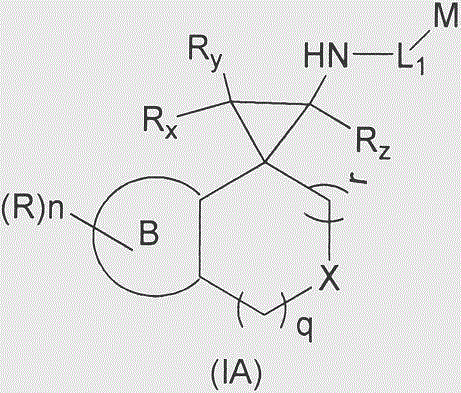
Cyclopropylamine spiro(hetero)cyclic compound, and pharmaceutical composition and application thereof
A technology for compounds and prodrugs, which can be used in drug combinations, preparation of amino compounds, preparation of organic compounds, etc., and can solve problems such as adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0170] Embodiment 1: the synthesis of compound I-1
[0171]
[0172] Step 1: Compound II-1-1
[0173] Dissolve p-N-tert-butoxycarbonylpiperidone (76mg, 0.38mmol), intermediate II-1 (60mg, 0.38mmol) and glacial acetic acid (23mg, 0.38mmol) in 1,2-dichloroethane (5mL ), stirred at room temperature for 1 hour, cooled in an ice bath, added sodium acetate borohydride (145mg, 0.68mmol), stirred at room temperature overnight, the reaction solution was quenched with dichloromethane, and the organic phase was washed successively with 1M citric acid solution and saturated brine , the reaction solution was concentrated and purified by silica gel column chromatography (dichloromethane / methanol=20 / 1) to obtain compound II-1-1 (20 mg, yield: 15%) as an oily liquid.
[0174] m / z: [M+H] + 343
[0175] Step 2: Compound I-1
[0176] Compound II-1-1 (20mg, 0.06mmol) and 2,6-lutidine (13mg, 0.12mmol) were dissolved in anhydrous dichloromethane (1mL), and trifluoromethanesulfon was added dr...
Embodiment 2
[0179] Example 2: Using the synthesis method of compound I-1, and corresponding aldehydes or ketones to synthesize stereoisomer mixtures I-2 to I-4:
[0180]
Embodiment 3
[0181] Embodiment 3: the synthesis of compound 1-5
[0182]
[0183] Dissolve 1-benzyl-4-piperidinecarbaldehyde (64 mg, 0.31 mmol), intermediate II-1 (50 mg, 0.31 mmol) and 2 drops of glacial acetic acid in 1,2-dichloroethane (1 mL), room temperature Stir for 1 hour, cool in an ice bath, add sodium acetate borohydride (197mg, 0.93mmol), stir at room temperature for 16 hours, dilute the reaction solution with dichloromethane (20mL), use saturated sodium bicarbonate solution (10mLx2), saturated It was washed with brine, dried over anhydrous sodium sulfate, concentrated by filtration and purified by silica gel column chromatography (dichloromethane / methanol=20) to obtain compound I-5 (25 mg, yield: 22%) as a yellow oil.
[0184] m / z: [M+H] + 347
[0185] 1 HNMR (DMSO-d 6 , 400Hz): δ7.14-7.33(m, 8H), 7.04-7.14(m, 1H), 3.44(s, 2H), 2.23-2.93(m, 5H), 1.45-2.57(m, 12H), 1.06 -1.17 (m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com