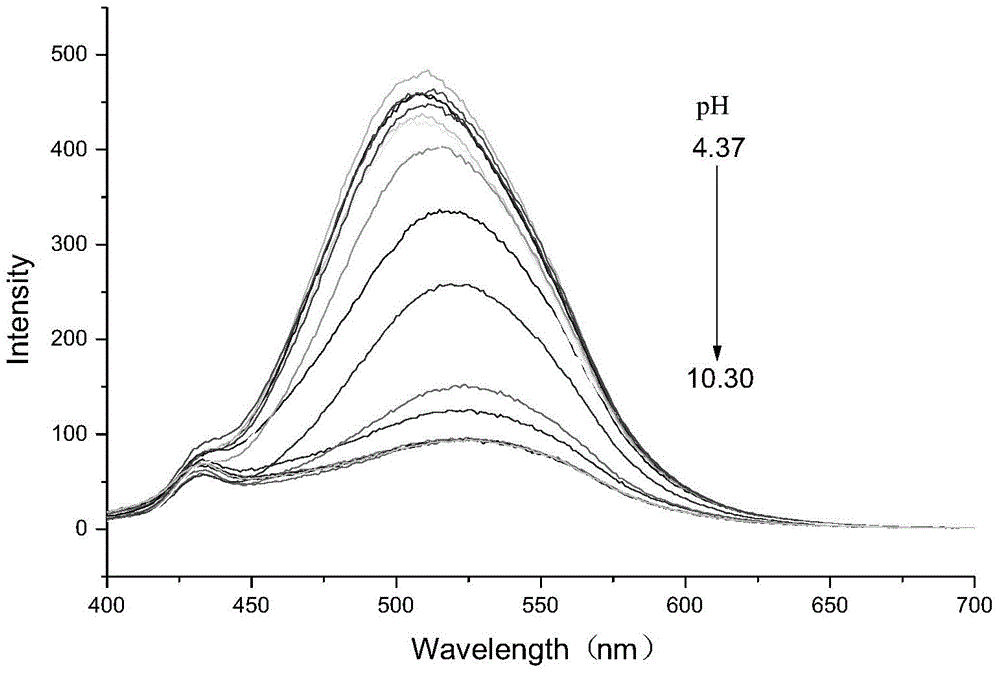
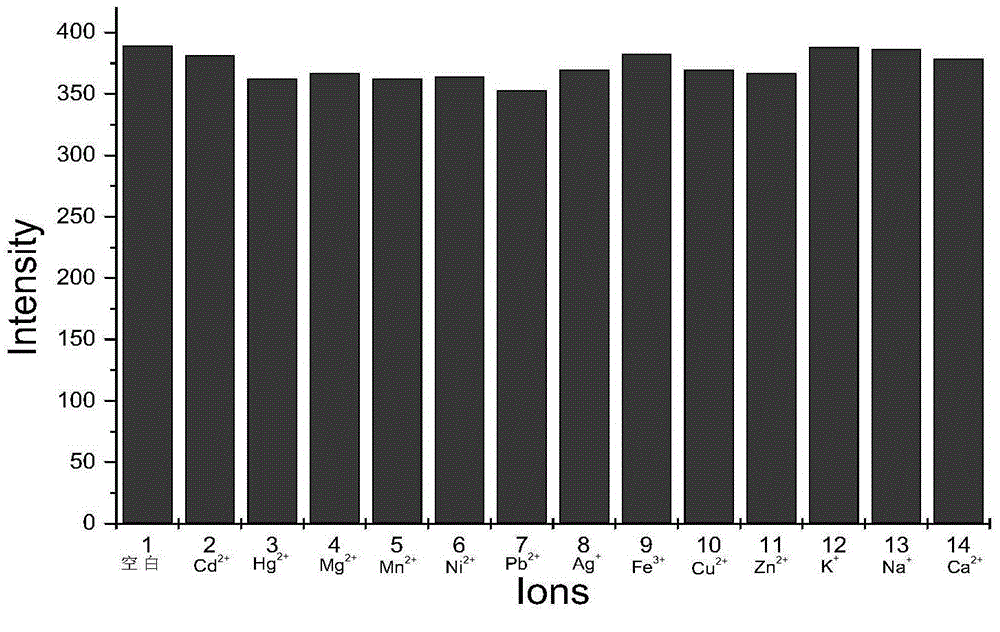
PH fluorescent probe and application of same
A probe and synthesis method technology, applied in the field of pH fluorescent probes, can solve the problems of small Stokes displacement and excitation light interference, and achieve the effects of reducing interference, high sensitivity and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A pH fluorescent probe, its chemical name is: iodide 1-methyl-2-(4-hydroxyl-3-methoxy) styryl pyridinium salt, its chemical structural formula is as follows:
[0023]
[0024] The synthetic steps of above-mentioned compound are as follows:
[0025] Weigh 0.235g (1.0mmol) 1,2-lutidine iodide and 0.316g vanillin in a 50ml round bottom flask, add 20ml n-butanol, add 0.01g piperazine, heat the mixed solution to reflux, and react 45min. After the reaction was detected by TLC, it was cooled to room temperature, and a large amount of solids were precipitated. After suction filtration under reduced pressure, n-butanol was recrystallized to obtain 0.215 g of yellow solids, with a yield of 58.3%.
[0026] Melting point determination: mp: 258-261°C
[0027] Infrared spectrometry: TR (KBr, cm -1 )3439, 3103, 1634, 1618, 1594, 1566, 1522, 1247, 1128
[0028] H NMR spectrum determination: 1 HNMR (400MH Z , DMSO): δ9.77 (s, 1H), δ8.88 (d, J=6.4H Z , 1H), δ8.49 (d, J=7.6H Z ...
Embodiment 2
[0033] Weigh 0.235g of 1,2-lutidine iodide and 0.304g of vanillin into a 50ml round bottom flask, add 20ml of n-butanol, add 0.01g of piperazine, heat the mixed solution to reflux, and react for 30min. After the reaction was detected by TLC, it was cooled to room temperature, a large amount of solids were precipitated, filtered under reduced pressure, and n-butanol was recrystallized to obtain 0.214 g of a yellow solid. Yield 58.0%.
[0034] The melting point determination of product, infrared spectrum determination, proton nuclear magnetic resonance spectrum determination are all with embodiment 1.
Embodiment 3
[0036] Weigh 0.235g of 1,2-lutidine iodide and 0.456g of vanillin into a 50ml round bottom flask, add 25ml of n-butanol, add 0.02g of piperazine, heat the mixed solution to reflux, and react for 60min. After the reaction was detected by TLC, it was cooled to room temperature, and a large amount of solids were precipitated, filtered under reduced pressure, and n-butanol was recrystallized to obtain 0.215 g of a yellow solid. Yield 58.3%.
[0037] The melting point determination of product, infrared spectrum determination, proton nuclear magnetic resonance spectrum determination are all with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com