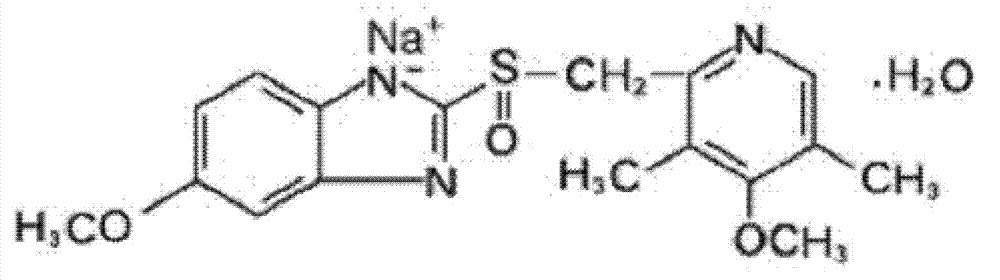
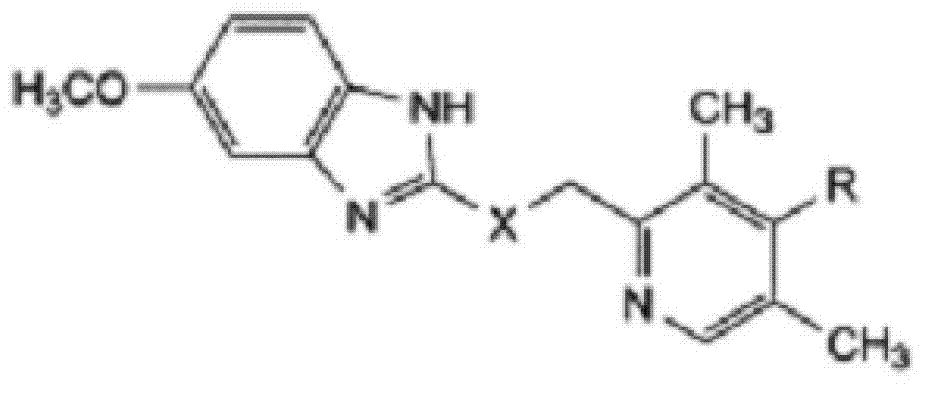
Omeprazole sodium and preparation method
A technology of omeprazole and sodium iodide, which is applied in the field of medicine, can solve the problems of long reaction time, low yield, and poor purity, and achieve the effects of shortening the reaction time, good product purity, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole
[0067] 180 grams (1mol) of 2-mercapto-5-methoxyl-1H-benzimidazole and 222 grams (1mol) of 2-chloromethyl-3,5-dimethyl-4-methoxypyridinium acid salt was added to 5 liters of ethanol and acetone mixed solvent (V:V=3:1), which contained 80 grams (2mol) of sodium hydroxide, while adding 5 grams (0.033mol) of sodium iodide, the reaction mixture was Heat to reflux for 1.5 hours, cool to room temperature, filter to remove insoluble matter, distill off most of the solvent from the filtrate under reduced pressure, add 3 liters of ethyl acetate to the residue to dissolve, wash twice with 1 liter of water, and dry the organic phase with anhydrous sodium sulfate , filtered, concentrated, and 400ml of acetone was added to the residue. The organic phase was frozen to 0°C, and a solid precipitated overnight, and was filtered to obtain 315.8 g of off-white solid 5-methoxy-2-[(4-...
Embodiment 2
[0068] Embodiment 2: preparation omeprazole
[0069] 5-methoxyl-2-[(4-methoxyl-3,5-dimethylpyridin-2-yl)methylthio]-1H-benzimidazole prepared in Example 1 was added to 1500ml of Ethyl acetate, cool the reaction system to -25°C, maintain the reaction temperature, slowly add peracetic acid (the amount is 5-methoxy-2-[(4-methoxy-3,5-dimethyl 1.2 times the molar weight of pyridin-2-yl)methylthio]-1H-benzimidazole) and lactic acid (the amount is 5-methoxy-2-[(4-methoxy-3,5-dimethyl basepyridin-2-yl)methylthio]-1H-benzimidazole molar weight of 0.2 times) of the mixture, continue to react for 0.5 hours after adding, while adjusting the pH value of the reaction system with 10% sodium hydroxide, maintain At pH=8, a precipitate precipitated, filtered, washed the filter cake with water, dried, and then recrystallized with methanol to obtain omeprazole as a white solid, yield: 94.8%.
[0070] [HPLC method B] of the present invention is used to measure the content of impurity C (unoxid...
Embodiment 21
[0071] Example 21: Preparation of Omeprazole
[0072] With reference to the method of Example 2 above, the difference is that the amount of lactic acid is 5-methoxy-2-[(4-methoxy-3,5-dimethylpyridin-2-yl)methylthio]-1H- 0.1 times the molar amount of benzimidazole. 【HPLC method B】Measurement results: the impurity C content is 0.004%, the impurity D content is 0.016%, and the HPLC purity is greater than 99.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com